Volume 6, Issue 1 (3-2021)
CJHR 2021, 6(1): 21-28 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Abouee-Mehrizi A. A National Survey of Iranian Academicians’ Attitudes Towards COVID-19 Vaccination. CJHR 2021; 6 (1) :21-28
URL: http://cjhr.gums.ac.ir/article-1-175-en.html
URL: http://cjhr.gums.ac.ir/article-1-175-en.html
Department of Occupational Health Engineering, School of Health, Tabriz University of Medical Sciences, Tabriz, Iran. , aramirreza818@gmail.com
Full-Text [PDF 1100 kb]
(502 Downloads)
| Abstract (HTML) (1658 Views)
Full-Text: (829 Views)
1. Introduction
Coronavirus Disease 2019 (COVID-19) is a serious threat to human health and life that has spread in many countries since 2020 [1]. Due to its rapid spread, it should be considered in both medical care and prevention strategies [2]. The production of vaccines for COVID-19 has been considered the most promising strategy and was the main target by many reputable pharmaceutical companies around the world since its inception [3]. Due to the length of the production process of the COVID-19 vaccine and the complexity of the manufacturing process, people’s attitudes about the COVID-19 vaccine are very different and significant [4]. Attention to the attitude of individuals regarding the COVID-19 vaccine in different communities should be considered when making and distributing this vaccine [5, 6]. The decline in public confidence in public health organizations has increased with the outbreak of COVID-19 [7].
Today, with the rise of public awareness of health issues, people’s perceptions of pharmaceutical companies have changed [8, 9]. The economic profitability of pharmaceutical companies, the increasing use of chemical and industrial raw materials, and bioterrorism have raised concerns about the use of chemical drugs and vaccines [10]. On the other hand, the remarkable advancement of pharmaceutical and medical sciences has raised more hopes for the cure of diseases in societies [11].
Considering the high number of people with COVID-19 and the number of deaths from the disease, many people are expected to have hope for access to vaccines and drugs to treat the disease as soon as possible [12]. Therefore, it is necessary to pay attention to people’s attitudes towards the COVID-19 vaccine, which will probably be produced in the near future. Because the academicians and faculty members are considered to be the frontline of knowledge building in the community influencing the value of health in other public members, the aim of this study was to investigate the attitudes of Iranian academicians and health professionals about the COVID-19 vaccine.
Today, with the rise of public awareness of health issues, people’s perceptions of pharmaceutical companies have changed [8, 9]. The economic profitability of pharmaceutical companies, the increasing use of chemical and industrial raw materials, and bioterrorism have raised concerns about the use of chemical drugs and vaccines [10]. On the other hand, the remarkable advancement of pharmaceutical and medical sciences has raised more hopes for the cure of diseases in societies [11].
Considering the high number of people with COVID-19 and the number of deaths from the disease, many people are expected to have hope for access to vaccines and drugs to treat the disease as soon as possible [12]. Therefore, it is necessary to pay attention to people’s attitudes towards the COVID-19 vaccine, which will probably be produced in the near future. Because the academicians and faculty members are considered to be the frontline of knowledge building in the community influencing the value of health in other public members, the aim of this study was to investigate the attitudes of Iranian academicians and health professionals about the COVID-19 vaccine.
2. Materials and Methods
Study design and study population
This study was a cross-sectional study carried out in a sample of Iranian academicians and health professionals for three months from the beginning of September 2020 to the beginning of November 2020. According to the previous estimate of 19% for mistrust about vaccines [13], considering a 95% confidence interval, 4% margin of error, and 20% non-response rate, a minimum number of 384 people was calculated for the sample size. All researchers of Iranian universities of medical sciences who lived in different cities in Iran graduated with Bachelor’s, Master’s, PhD, or Postdoctoral degrees in health and medical sciences, were included in the study. Exclusion criteria were researchers who did not live in Iran. Moreover, people who answered less than 95% of the questions of the questionnaire for any reason or entered irrelevant information were excluded from the study.
After preparing the questionnaire, it was made available to the targeted subjects online. The link to the questionnaire along with a guideline was emailed to each individual. The email addresses of the subjects were collected through scientific resumes registered in the research systems of Iranian universities of medical sciences, which were available online through the website of each university. The informed consent form was completed electronically by the participants at the beginning of the study.
The number of universities, to which e-mail invitations were sent to researchers of those universities included all medical universities and subdivisions of the Ministry of Health and Medical Education of Iran. The number of these universities was 68. First, the designed questionnaire was prepared through a public link in Google docs. Then, the link to the questionnaire was sent to the researchers via email, which was in the Pazhoohan research system or the professors’ personal resumes in the link of their university. Researchers were screened through the Pazhoohan research system. One week after the first email was sent, a reminder email was sent to all professors. To have the random sample and have the results generalizable to the whole population, researchers were randomly selected whose first and last letters of their last names were the first and second letters of Persian letters.
Study instrument
The questionnaire was designed based on a study on mapping global trends in vaccine confidence by de Figueiredo et al. [14]. The English questionnaire was first translated into Persian by the researchers and for back translation strategy, the translated text was transmitted to ten professors and experts in public health to be translated back into English. Then, the text translated into English was translated into Persian and returned to the same experts and also experts for translation into English.
The content validity of the questionnaire was approved by calculating the Content Validity Ratio (CVR). A panel of researchers, including six statisticians and epidemiologists, were invited to rate the items in terms of clarity, relevance, and necessity. Some items needed revision and the final CVR was 0.99. The reliability of the questionnaire was assessed using Cronbach’s alpha method by SPSS software. Accordingly, 100 cases of the surveyed population were randomly distributed and the results were compared to determine the reliability of the questionnaire using Cronbach’s alpha. For example, for the variables related to the “Source of trust items”, the Cronbach’s alpha was 0.722 and for the variables related to the “Vaccine confidence”, the Cronbach’s alpha was 0.731. The Cronbach’s alpha above 0.7 is statistically acceptable for the reliability of the questionnaire.
The questionnaire consisted of 4 parts. The first part was related to the explanation of the purpose of the study and obtaining informed consent to participate in the study. The second part included questions about participants’ demographic information. The third section included questions about medical history information, and the fourth section included questions related to the preparation and production of the COVID-19 vaccine consisted of 5 subsections, including A: Vaccine confidence, B: Vaccine uptake, C: Time estimation, D: Source of trust, and E: Information seeking.
Statistical analysis
Data were described as frequency and percent. The Chi-square statistical method was used to compare responses in different groups based on demographic characteristics (gender, level of education, and field of study). IBM SPSS software (version 25) was used for statistical analysis. The statistical significance level was considered at 0.05.
This study was a cross-sectional study carried out in a sample of Iranian academicians and health professionals for three months from the beginning of September 2020 to the beginning of November 2020. According to the previous estimate of 19% for mistrust about vaccines [13], considering a 95% confidence interval, 4% margin of error, and 20% non-response rate, a minimum number of 384 people was calculated for the sample size. All researchers of Iranian universities of medical sciences who lived in different cities in Iran graduated with Bachelor’s, Master’s, PhD, or Postdoctoral degrees in health and medical sciences, were included in the study. Exclusion criteria were researchers who did not live in Iran. Moreover, people who answered less than 95% of the questions of the questionnaire for any reason or entered irrelevant information were excluded from the study.
After preparing the questionnaire, it was made available to the targeted subjects online. The link to the questionnaire along with a guideline was emailed to each individual. The email addresses of the subjects were collected through scientific resumes registered in the research systems of Iranian universities of medical sciences, which were available online through the website of each university. The informed consent form was completed electronically by the participants at the beginning of the study.
The number of universities, to which e-mail invitations were sent to researchers of those universities included all medical universities and subdivisions of the Ministry of Health and Medical Education of Iran. The number of these universities was 68. First, the designed questionnaire was prepared through a public link in Google docs. Then, the link to the questionnaire was sent to the researchers via email, which was in the Pazhoohan research system or the professors’ personal resumes in the link of their university. Researchers were screened through the Pazhoohan research system. One week after the first email was sent, a reminder email was sent to all professors. To have the random sample and have the results generalizable to the whole population, researchers were randomly selected whose first and last letters of their last names were the first and second letters of Persian letters.
Study instrument
The questionnaire was designed based on a study on mapping global trends in vaccine confidence by de Figueiredo et al. [14]. The English questionnaire was first translated into Persian by the researchers and for back translation strategy, the translated text was transmitted to ten professors and experts in public health to be translated back into English. Then, the text translated into English was translated into Persian and returned to the same experts and also experts for translation into English.
The content validity of the questionnaire was approved by calculating the Content Validity Ratio (CVR). A panel of researchers, including six statisticians and epidemiologists, were invited to rate the items in terms of clarity, relevance, and necessity. Some items needed revision and the final CVR was 0.99. The reliability of the questionnaire was assessed using Cronbach’s alpha method by SPSS software. Accordingly, 100 cases of the surveyed population were randomly distributed and the results were compared to determine the reliability of the questionnaire using Cronbach’s alpha. For example, for the variables related to the “Source of trust items”, the Cronbach’s alpha was 0.722 and for the variables related to the “Vaccine confidence”, the Cronbach’s alpha was 0.731. The Cronbach’s alpha above 0.7 is statistically acceptable for the reliability of the questionnaire.
The questionnaire consisted of 4 parts. The first part was related to the explanation of the purpose of the study and obtaining informed consent to participate in the study. The second part included questions about participants’ demographic information. The third section included questions about medical history information, and the fourth section included questions related to the preparation and production of the COVID-19 vaccine consisted of 5 subsections, including A: Vaccine confidence, B: Vaccine uptake, C: Time estimation, D: Source of trust, and E: Information seeking.
Statistical analysis
Data were described as frequency and percent. The Chi-square statistical method was used to compare responses in different groups based on demographic characteristics (gender, level of education, and field of study). IBM SPSS software (version 25) was used for statistical analysis. The statistical significance level was considered at 0.05.
3. Results
Of 16500 online invitations sent to the researchers from different medical and health universities in Iran, 918 cases filled the questionnaire completely except for the monthly income question, which was an optional item (participation rate=5%). The Mean±SD age of participants was 35.3±10.0 years (age range: 23-67 years). More than half of the participants (55.7%) were female. Moreover, a doctoral degree was the most common academic degree reported by the participants (51.50%), followed by Master’s (30.80%), and Bachelor’s degrees (14.50%). Medical and health sciences were the most field of the study reported by participants. Lower than half of the participants had remarkable symptoms of COVID-19 infection or had a taken the COVID-19 test. Among 320 participants who reported symptoms of COVID-19 infection, 60% had weakness and lethargy, 15% had a dry cough, 12% had a fever, 10% has diarrhea, and 3% had nausea. Table 1 shows demographic and educational characteristics and medical history of study participants.
.jpg)
The distribution of participants from different universities in Iran is illustrated in Figure 1.
.jpg)
Tehran, Razavi-Khorasan, and East-Azerbaijan were provinces with the most participants.
Also, 31.6% and 38.2% of participants agreed that the COVID-19 vaccine is safe and effective, respectively (Figure 2).
.jpg)
The majority of participants (67.2%) responded that the country has to provide the vaccine for free rather than paying the cost of the vaccine. Moreover, 73.0% (n=670) preferred the COVID-19 vaccine made in a foreign country to the COVID-19 vaccine made in their own country. Among people who chose the COVID-19 vaccine made in a foreign country, 45.9 % chose the COVID-19 vaccine made in the USA (Figure 3).
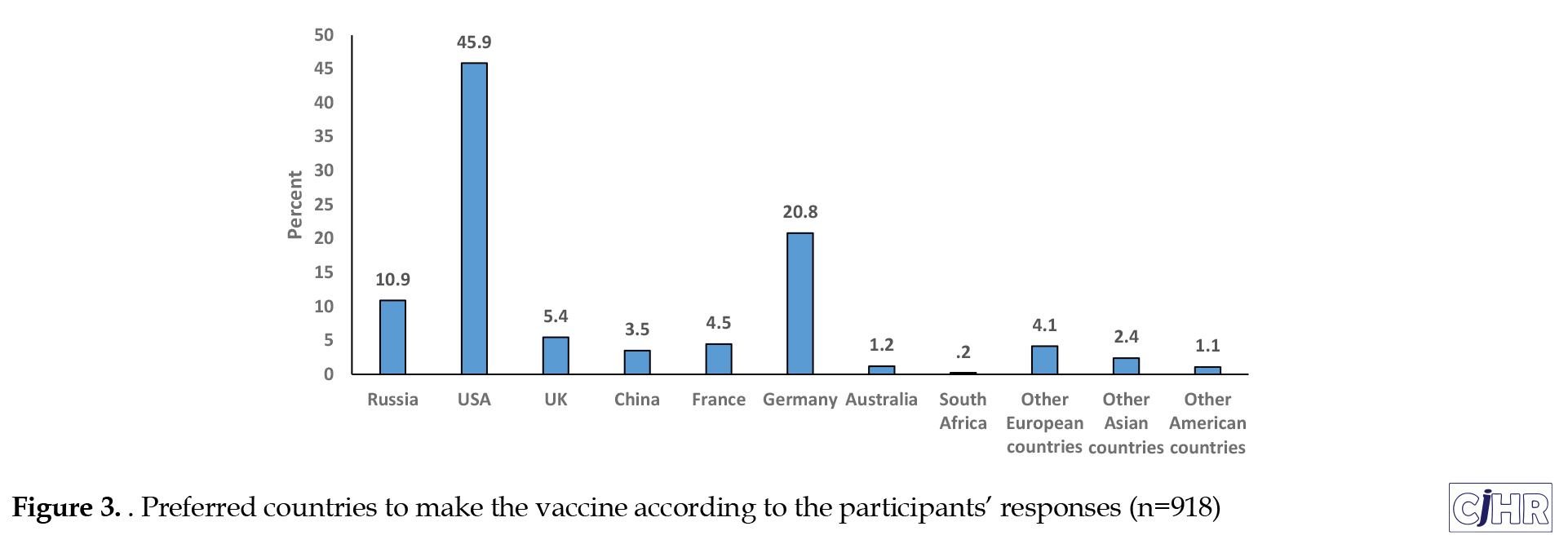
Although 46.3 % were not sure about the effectiveness and safety of the COVID-19 vaccine, 68.3 % of the participants were volunteer to get the COVID-19 vaccine presented by the government for themselves. According to the results, 49% (450 people) stated that they had no children. In other cases, 51% (468 people) had at least one child. Among participants, who had any child, 43.81% did not choose to take their children to a health center or other vaccine center to get the COVID-19 vaccine. Elderly people were chosen by 59.30% of participants to take the COVID-19 vaccine compared with different age groups in the society. Also, 59.6% believed that the COVID-19 vaccine will be available before the end of 2021. However, near 25.1 % believed that the COVID-19 vaccine cannot be available before the end of 2021, and 15.3% thought that the COVID-19 vaccine will be available after the beginning of 2022. Almost 78% of participants tried to get any information about the COVID-19 vaccine in the past 30 days from the date of filling in the questionnaire. According to Table 2, the government and traditional healers had not a trustworthy source for the people. Nevertheless, most people believed in information about the COVID-19 vaccine, which was reported by the health workers, such as nurses and doctors (Table 2).
.jpg)
Attitudes towards COVID-19 vaccination were significantly associated with participants’ gender and level of education. Females more disagreed (15.3%) with the effectiveness of the COVID-19 vaccine compared with males (9.1%, P=0.02). People with Ph.D. and master’s degrees more disagreed (14.2%) with the effectiveness of the vaccine compared with those with diploma and bachelor academic degrees (6.8%, P=0.01).
.jpg)
The distribution of participants from different universities in Iran is illustrated in Figure 1.
.jpg)
Tehran, Razavi-Khorasan, and East-Azerbaijan were provinces with the most participants.
Also, 31.6% and 38.2% of participants agreed that the COVID-19 vaccine is safe and effective, respectively (Figure 2).
.jpg)
The majority of participants (67.2%) responded that the country has to provide the vaccine for free rather than paying the cost of the vaccine. Moreover, 73.0% (n=670) preferred the COVID-19 vaccine made in a foreign country to the COVID-19 vaccine made in their own country. Among people who chose the COVID-19 vaccine made in a foreign country, 45.9 % chose the COVID-19 vaccine made in the USA (Figure 3).

Although 46.3 % were not sure about the effectiveness and safety of the COVID-19 vaccine, 68.3 % of the participants were volunteer to get the COVID-19 vaccine presented by the government for themselves. According to the results, 49% (450 people) stated that they had no children. In other cases, 51% (468 people) had at least one child. Among participants, who had any child, 43.81% did not choose to take their children to a health center or other vaccine center to get the COVID-19 vaccine. Elderly people were chosen by 59.30% of participants to take the COVID-19 vaccine compared with different age groups in the society. Also, 59.6% believed that the COVID-19 vaccine will be available before the end of 2021. However, near 25.1 % believed that the COVID-19 vaccine cannot be available before the end of 2021, and 15.3% thought that the COVID-19 vaccine will be available after the beginning of 2022. Almost 78% of participants tried to get any information about the COVID-19 vaccine in the past 30 days from the date of filling in the questionnaire. According to Table 2, the government and traditional healers had not a trustworthy source for the people. Nevertheless, most people believed in information about the COVID-19 vaccine, which was reported by the health workers, such as nurses and doctors (Table 2).
.jpg)
Attitudes towards COVID-19 vaccination were significantly associated with participants’ gender and level of education. Females more disagreed (15.3%) with the effectiveness of the COVID-19 vaccine compared with males (9.1%, P=0.02). People with Ph.D. and master’s degrees more disagreed (14.2%) with the effectiveness of the vaccine compared with those with diploma and bachelor academic degrees (6.8%, P=0.01).
4. Discussion
Vaccination is known as one of the most effective ways to prevent many diseases [15]. People’s attitudes toward using a vaccine for a disease depend on several factors, such as the epidemic or fatality of the disease [14]. In previous studies, people’s attitudes toward the use of vaccines, in general, have been mentioned [14]. However, due to the complexities of producing effective drugs and vaccines for the COVID-19, people may have different opinions about vaccines or drugs for the disease.
In a study conducted by de Figueiredo et al., it was estimated that confidence in the safety and effectiveness of vaccines decreased in Afghanistan, Indonesia, Pakistan, the Philippines, and South Korea between 2015 and 2019 [14]. Moreover, disagreement on the safety of vaccines significantly increased in respondents between 2015 and 2019 in Azerbaijan, Afghanistan, Indonesia, Nigeria, Pakistan, and Serbia [14]. Paul et al. reported that 16% of participants in the study had high levels of distrust toward the COVID-19 vaccine in the UK. Indefinite attitudes towards vaccination were noticeable among people with lower annual income, poor levels of education, lower knowledge of COVID-19, and lower agreement with government COVID-19 strategies in the UK. Moreover, 14% of people stated that they will not receive a COVID-19 vaccine [13].
The current study indicated that attitudes toward the COVID-19 vaccine in Iranian researchers were significantly associated with some studied variables. More than half doubted that the vaccine will be safe and effective. Differences in opinion about the safety and efficacy of the COVID-19 vaccine and the time of vaccine availability may be due to the novelty of the coronavirus, multiple genetic mutations in the virus, lack of definitive treatment, and other unknown problems caused by this virus. This study indicated that the contribution and attitude of Iranians were not the same in different cities in Iran. The cause of this matter might be related to different demographic parameters, such as economic, social, or cultural characteristics, and beyond the scientific information of the participants. This importance could be a sign of the need for improving information and attitude of people not only among different countries but also inside of countries.
Nevertheless, in some cases, people agreed on the vaccine preparation and uptake. For example, most people believed that the elderly should be the first group to receive the vaccine. People also relied heavily on the medical staff for vaccine information compared with the government and traditional medicine. Most people wanted to get the vaccine produced in a foreign country. A large number of participants chose the United States, Germany, and Russia as vaccine-producing countries.
Reiter et al. showed that about 69% of American participants would like to use the COVID-19 vaccine in the USA in 2020. This study indicated that black people have different views on the use of the COVID-19 vaccine compared with white people living in the United States [4]. This issue indicated that different cultures inside a country might have dissimilar attitudes toward the COVID-19 vaccine as mentioned in the current study in Iran.
In contrast to the current study, Reuben et al. showed that 79.5% of the people in north-central Nigeria have a positive attitude towards the health measures provided by the government in 2020. However, nearly 50% of respondents believed that the government should make more efforts to control COVID-19 [5]. Reuben et al. and the current study indicated that Nigerians and Iranians had not sufficient trust in their governments regarding the COVID-19 vaccine, and their governments need to build more trust in this regard.
Neumann-Böhme et al. showed that 55% of people living in Europe thought that the COVID-19 vaccine has potential adverse effects and 15% are unsure of the safety of the COVID-19 vaccine; however, 73.6 % of them tend to get the vaccine for themselves. They found that the COVID-19 has affected the lives of Europeans to such an extent that people in these countries, despite their doubts about the safety of the COVID-19 vaccine, prefer to receive the vaccine [16].
Fisher et al. indicated that 57.6% of Americans intended to be vaccinated, 31.6% had not no specific idea, and 10.8% would not be vaccinated. Their idea was associated with their age, race, and educational level. People stated that they need more information to trust the government and health organizations [17].
In this study, 5% accepted the invitation and participated in the study. The novelty of the COVID-19 and the lack of sufficient knowledge about the disease might be some of the reasons for the non-cooperation of the majority of those invited to participate in the study. On the other hand, some may have assumed that the study was affiliated with the Iranian government and refused to participate in the study for political reasons.
The results obtained from the current study compared with the above-mentioned studies indicated that although Europeans, Americans, and Iranians were not sure about the safety and effectiveness of the COVID-19 vaccine, they are intended to receive the vaccine. This point might result from the high-speed spreading of the virus and the adverse effects caused by this virus on all people around the world.
In a study conducted by de Figueiredo et al., it was estimated that confidence in the safety and effectiveness of vaccines decreased in Afghanistan, Indonesia, Pakistan, the Philippines, and South Korea between 2015 and 2019 [14]. Moreover, disagreement on the safety of vaccines significantly increased in respondents between 2015 and 2019 in Azerbaijan, Afghanistan, Indonesia, Nigeria, Pakistan, and Serbia [14]. Paul et al. reported that 16% of participants in the study had high levels of distrust toward the COVID-19 vaccine in the UK. Indefinite attitudes towards vaccination were noticeable among people with lower annual income, poor levels of education, lower knowledge of COVID-19, and lower agreement with government COVID-19 strategies in the UK. Moreover, 14% of people stated that they will not receive a COVID-19 vaccine [13].
The current study indicated that attitudes toward the COVID-19 vaccine in Iranian researchers were significantly associated with some studied variables. More than half doubted that the vaccine will be safe and effective. Differences in opinion about the safety and efficacy of the COVID-19 vaccine and the time of vaccine availability may be due to the novelty of the coronavirus, multiple genetic mutations in the virus, lack of definitive treatment, and other unknown problems caused by this virus. This study indicated that the contribution and attitude of Iranians were not the same in different cities in Iran. The cause of this matter might be related to different demographic parameters, such as economic, social, or cultural characteristics, and beyond the scientific information of the participants. This importance could be a sign of the need for improving information and attitude of people not only among different countries but also inside of countries.
Nevertheless, in some cases, people agreed on the vaccine preparation and uptake. For example, most people believed that the elderly should be the first group to receive the vaccine. People also relied heavily on the medical staff for vaccine information compared with the government and traditional medicine. Most people wanted to get the vaccine produced in a foreign country. A large number of participants chose the United States, Germany, and Russia as vaccine-producing countries.
Reiter et al. showed that about 69% of American participants would like to use the COVID-19 vaccine in the USA in 2020. This study indicated that black people have different views on the use of the COVID-19 vaccine compared with white people living in the United States [4]. This issue indicated that different cultures inside a country might have dissimilar attitudes toward the COVID-19 vaccine as mentioned in the current study in Iran.
In contrast to the current study, Reuben et al. showed that 79.5% of the people in north-central Nigeria have a positive attitude towards the health measures provided by the government in 2020. However, nearly 50% of respondents believed that the government should make more efforts to control COVID-19 [5]. Reuben et al. and the current study indicated that Nigerians and Iranians had not sufficient trust in their governments regarding the COVID-19 vaccine, and their governments need to build more trust in this regard.
Neumann-Böhme et al. showed that 55% of people living in Europe thought that the COVID-19 vaccine has potential adverse effects and 15% are unsure of the safety of the COVID-19 vaccine; however, 73.6 % of them tend to get the vaccine for themselves. They found that the COVID-19 has affected the lives of Europeans to such an extent that people in these countries, despite their doubts about the safety of the COVID-19 vaccine, prefer to receive the vaccine [16].
Fisher et al. indicated that 57.6% of Americans intended to be vaccinated, 31.6% had not no specific idea, and 10.8% would not be vaccinated. Their idea was associated with their age, race, and educational level. People stated that they need more information to trust the government and health organizations [17].
In this study, 5% accepted the invitation and participated in the study. The novelty of the COVID-19 and the lack of sufficient knowledge about the disease might be some of the reasons for the non-cooperation of the majority of those invited to participate in the study. On the other hand, some may have assumed that the study was affiliated with the Iranian government and refused to participate in the study for political reasons.
The results obtained from the current study compared with the above-mentioned studies indicated that although Europeans, Americans, and Iranians were not sure about the safety and effectiveness of the COVID-19 vaccine, they are intended to receive the vaccine. This point might result from the high-speed spreading of the virus and the adverse effects caused by this virus on all people around the world.
5. Conclusion
The results of this study showed that researchers in Iranian universities of medical sciences do not have the necessary confidence and readiness to receive the COVID-19 vaccine in different provinces in Iran. Considering that this group is an educated group of the society, we can expect that people have more doubts about the vaccine. Therefore, before distributing the vaccine, people should be informed about the various aspects of using the COVID-19 vaccine because people may not be willing to get the COVID-19 vaccine despite the availability of the vaccine.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Ethics Committee of the Iranian Occupational Health Engineering Student Scientific Committee, Tabriz (Code: OHSIRST.001.5.2021).
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Conflict of interest
The author declared no conflict of interest.
References
- Clark A, Jit M, Warren-Gash C, Guthrie B, Wang HHX, Mercer SW, et al. Global, regional, and national estimates of the population at increased risk of severe COVID-19 due to underlying health conditions in 2020: A modelling study. Lancet Glob Health. 2020; 8(8):e1003-17. [DOI:10.1016/j.envres.2020.110129] [PMID] [PMCID]
- Copat C, Cristaldi A, Fiore M, Grasso A, Zuccarello P, Signorelli SS, et al. The role of air pollution (PM and NO2) in COVID-19 spread and lethality: A systematic review. Environ Res. 2020; 191:110129. [DOI:10.1016/j.envres.2020.110129] [PMID] [PMCID]
- Shin MD, Shukla S, Chung YH, Beiss V, Chan SK, Ortega-Rivera OA, et al. COVID-19 vaccine development and a potential nanomaterial path forward. Nat Nanotechnol. 2020; 15(8):646-55. [DOI:10.1038/s41565-020-0737-y] [PMID]
- Reiter PL, Pennell ML, Katz ML. Acceptability of a COVID-19 vaccine among adults in the United States: How many people would get vaccinated? Vaccine. 2020; 38(42):6500-7. [DOI:10.1016/j.vaccine.2020.08.043] [PMID] [PMCID]
- Reuben RC, Danladi MMA, Saleh DA, Ejembi PE. Knowledge, attitudes and practices towards COVID-19: An epidemiological survey in North-Central Nigeria. J Community Health. 2021; 46(3):457-70. [DOI:10.1007/s10900-020-00881-1] [PMID] [PMCID]
- Sibley CG, Greaves LM, Satherley N, Wilson MS, Overall NC, Lee CHJ, et al. Effects of the COVID-19 pandemic and nationwide lockdown on trust, attitudes toward government, and well-being. Am Psychol. 2020; 75(5):618-30. [DOI:10.1037/amp0000662] [PMID]
- Nussbaumer-Streit B, Mayr V, Dobrescu AI, Chapman A, Persad E, Klerings I, et al. Quarantine alone or in combination with other public health measures to control COVID‐19: A rapid review. Cochrane Database Syst Rev. 2020; 4(4):CD013574. [DOI:10.1002/14651858.CD013574] [PMID] [PMCID]
- Balfour L, Kowal J, Corace KM, Tasca GA, Krysanski V, Cooper CL, et al. Increasing public awareness about hepatitis C: Development and validation of the brief hepatitis C knowledge scale. Scand J Caring Sci. 2009; 23(4):801-8. [DOI:10.1111/j.1471-6712.2008.00668.x] [PMID]
- Shankardass K, Lofters A, Kirst M, Quiñonez C. Public awareness of income-related health inequalities in Ontario, Canada. Int J Equity Health. 2012; 11:26. [DOI:10.1186/1475-9276-11-26] [PMID] [PMCID]
- Khatami M. Cancer statistics and concerns for safety of drugs or vaccines: Increased population of drug-dependent sick society! In: M. Khatami, editor. Inflammation, aging and cancer. New York: Springer; 2017. pp. 213-60. [DOI:10.1007/978-3-319-66475-0_5]
- Sela M, Arnon R, Schechter B. Therapeutic vaccines: Realities of today and hopes for the future. Drug Ddiscov Today. 2002; 7(12):664-73. [DOI:10.1016/S1359-6446(02)02296-1]
- Patel SK, Pathak M, Tiwari R, Yatoo MI, Malik YS, Sah R, et al. A vaccine is not too far for COVID-19. J Infect Dev Ctries. 2020; 14(5):450-3. [DOI:10.3855/jidc.12744] [PMID]
- Paul E, Steptoe A, Fancourt D. Attitudes towards vaccines and intention to vaccinate against COVID-19: Implications for public health communications. Lancet Reg Health Eur. 2021; 1:100012. [DOI:10.1016/j.lanepe.2020.100012] [PMID] [PMCID]
- de Figueiredo A, Simas C, Karafillakis E, Paterson P, Larson HJ. Mapping global trends in vaccine confidence and investigating barriers to vaccine uptake: A large-scale retrospective temporal modelling study. Lancet. 2020; 396(10255):898-908. [DOI:10.1016/S0140-6736(20)31558-0] [PMID]
- Aguilar JC, Rodríguez EG. Vaccine adjuvants revisited. Vaccine. 2007; 25(19):3752-62. [DOI:10.1016/j.vaccine.2007.01.111] [PMID]
- Neumann-Böhme S, Varghese NE, Sabat I, Barros PP, Brouwer W, van Exel J, et al. Once we have it, will we use it? A European survey on willingness to be vaccinated against COVID-19. Eur J Health Econ. 2020; 21(7):977-82. [DOI:10.1007/s10198-020-01208-6] [PMID] [PMCID]
- Fisher KA, Bloomstone SJ, Walder J, Crawford S, Fouayzi H, Mazor KM. Attitudes toward a potential SARS-CoV-2 Vaccine: A survey of U.S. Adults. Ann Intern Med. 2020; 173(12):964-73. [DOI:10.7326/M20-3569] [PMID] [PMCID]
Article Type: Original Contributions |
Subject:
Public Health
Received: 2020/12/15 | Accepted: 2021/03/1 | Published: 2021/03/30
Received: 2020/12/15 | Accepted: 2021/03/1 | Published: 2021/03/30
Send email to the article author
| Rights and permissions | |
 | This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |









