Volume 8, Issue 3 (7-2023)
CJHR 2023, 8(3): 151-162 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Habokwesiga L, Lubowa N. Sodium Hypochlorite Diagnostic Performance compared to Ziehl-Nelseen method among Presumptive Tuberculosis Patients in Uganda. CJHR 2023; 8 (3) :151-162
URL: http://cjhr.gums.ac.ir/article-1-297-en.html
URL: http://cjhr.gums.ac.ir/article-1-297-en.html
1- Department of Biotechnical and Diagnostic Sciences, School of Biosecurity, Biotechnical and Laboratory Sciences, Makerere University, Kampala, Uganda. , hablabans@gmail.com
2- Department of Biotechnical and Diagnostic Sciences, College of Veterinary Medicine, Animal Resources and Biosecurity, Makerere University, Kampala, Uganda.
2- Department of Biotechnical and Diagnostic Sciences, College of Veterinary Medicine, Animal Resources and Biosecurity, Makerere University, Kampala, Uganda.
Full-Text [PDF 659 kb]
(129 Downloads)
| Abstract (HTML) (406 Views)
Full-Text: (113 Views)
1. Introduction
Tuberculosis remains a major public health problem, with an estimated 2 billion people being infected with Tubercle bacilli worldwide despite the fact that the causative organism was discovered more than 100 years ago [1]. In 2013, 9 million people fell ill with Tuberculosis (TB) and 1.5 million died from the disease. According to the World Health Organization (WHO) [2], low- and middle-income countries account for almost 95% of TB mortality. South-East Asia and the Western Pacific region saw the most new cases of TB, making up 56% of all new cases worldwide. With 280 new cases for every 100,000 people, Africa had the highest rate of new infections in 2013. In Uganda, tuberculosis prevalence rate was 0.2% [2]. However, between 2000 and 2003, TB diagnosis and treatment are thought to have saved an estimated 37 million lives [2]. This therefore shows that if additional and cheaper sensitive diagnostic methods like Concentration methods are put in place for TB early diagnosis, then Tuberculosis disease would easily be reduced to a low rate.
Diagnosis of Mycobacterium tuberculosis depends on examining the sputum for bacteria. The most effective way to find tubercle bacilli is through Mycobacterium culture, however it is incredibly time-consuming and needs extra safety precautions in laboratories. Serological methods lack sensitivity and specificity, making them ineffective in control programs. The nucleic acid amplification techniques are the most promising new methods for diagnosing TB quickly, but the technology is not suitable for control programs in developing nations [1]. In addition, fluorescent microscopy with Auramine-O or Rhodamine staining has been done but is not easily implemented in district health centers as compared to direct Ziehl Nelseen (ZN) microscopy which in turn has got shortcomings like decreased sensitivity.
HIV/TB co-infection makes sputum microscopy less sensitive [3]. The sodium hypochlorite concentration method, one of the safest concentration methods for boosting the sensitivity of direct microscopy for the detection of AFB, can be used to increase the sensitivity of sputum microscopy. The sensitivity of direct ZN microscopy can be greatly increased by sputum liquefaction with sodium hypochlorite (NaOCl/Jik) and concentration through centrifugation [4].
Related previous study findings [1] conducted in BLDEU’s Shri B.M.Patil Medical College, Hospital and Research Centre, India show that there has been a significant increase in sensitivity when sputum is concentrated with 5% sodium hypochlorite. Results show that out of 255 sputum samples analyzed, 25 sputum samples were positive for AFBs by direct smear microscopy while 84 were positive for AFBs in concentrated sputum samples. The ZN sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were 29%, 99%, 96% and 74% respectively with a 95% confidence interval, with use of 5% NaOCl method. Concentrating sputum with 5% NaOCl may improve ZN sensitivity hence improving diagnosis.
Diagnosis of Mycobacterium Tuberculosis remains a challenge in District Health Centers and Clinics in Uganda. Direct Ziehl-Nelseen staining method on sputum samples is the conventional Laboratory method for diagnosis of Tuberculosis. However, there are higher chances of missing TB positive cases due to insufficient sensitivity. This would eventually lead to TB transmission and hence mortality. There is a need of alternative ways of improving sensitivity of sputum microscopy. Therefore, the aim of this study was to find out whether use of Sodium Hypochlorite to concentrate sputum improves sensitivity compared to direct ZN microscopy. The general objective was to compare operating characteristics of NaOCl concentration method and direct Ziehl-Nelseen method on sputum samples for diagnosis of Tuberculosis. The specific objectives were: 1) To determine sensitivity and specificity of ZN on concentrated and unconcentrated sputum samples among TB presumptive patients attending Mulago Hospital. 2. To assess how clinical, sociodemographic, and sample characteristics affect concentrated sputum ZN positivity.
2. Materials and Methods
Study design
This was a cross-sectional study of the comparison of Sodium hypochlorite concentrated and direct Ziehl-Nelseen stained sputum smears processed in the period ranging from January-March 2016.
Study area
The study was conducted in TB ward laboratory, Mulago National Referral Hospital. Mulago Hospital is located three kilometers from Kampala city, the Ugandan capital city.
Target population
All adult TB suspect patients visiting the TB ward at Mulago Hospital in Kampala, Uganda made up the target group.
Sample
Adult patients attending TB ward in Mulago Hospital, Kampala, Uganda between January and March 2016. Early morning sputum samples from 114 patients attending Mulago Hospital TB ward from January to March 2016 of adult TB Presumptive patients of either sex, having a cough for three weeks or more, stayingwith people who have tested positive on a sputum smear regardless of how long they have had a cough, collected in well labeled and clean containers recommended for sputum sample collection with complete laboratory request forms and signed consent forms were received, analyzed and included in the study. Sputum samples without complete filled laboratory request forms and signed consent forms plus those collected in wrong containers were excluded from the study.
Sample size determination (Equation 1):
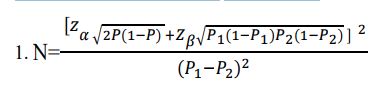
The sample size was determined according KP Suresh & S Chandrashekara’s formular [5].
Where, P1= Sensitivity of NaOCl,
P2= Sensitivity of direct ZN and P=
P1 =60.2% P2 =30.8% P=91% , When expressed as a proportion;
P1=0.602 P2=0.308 P=0.455
Zα is 1.96 and Zβ is 0.84 from the statistical tables. Substituting in the formular, N=49.75.
Minimum sample size (N) is 50 samples. However, for the purposes of increasing statistical power, a sample size of 114 samples was considered in this study.
Data collection methods
The clinical, socio-demographic data, ward, identification number, date, macroscopy and microscopy results of the patients were recorded. The questionnaire was used during collection of such information.
Laboratory procedures
Early morning sputum samples were taken in a sterile, leak-proof labelled container with a wide aperture by patients soon after they wake up and before any mouth wash was used. The patient was requested to cough deeply to produce a sputum specimen.
Two sets of sputum samples were collected and smears were prepared from each sample. The muco-purulent portion of the sputum was placed on a fresh, spotless, and grease free glass labeled slide for the first set, and it was then left to air dry. Dried smears were heat fixed and stained by ZN staining method. During this staining method, strong Carbolfuchsin stain was poured on the heat fixed slides on a staining rack and then heat was applied until vapor came. Slides with the stain were exposed for five minutes. After that, the slide was washed with fresh water and decolored for five minutes using 25% sulfuric acid. This was followed by washing and then counter stained with methylene blue stain for 2 minutes. Finally, the slides were washed and air dried ready for examination.
For the second sample, 1-2 ml of sputum was placed in disposable falcon tubes with a screw lid. The test tube was filled with an equal volume of 5% sodium hypochlorite and left at room temperature for 30 minutes. The test tube was shaken intermittently and about 8 ml of distilled water was added to the test tube. The test tube was then centrifuged at 3000 g for 15 minutes. After carefully discarding the supernatant, the sediments were used to make smears. Heat was used to fix the smears, and the ZN staining procedure was used to stain them similarly (Appendix:1).
Determining the sensitivity and specificity of NaOCl concentration and direct ZN methods
Dried ZN-stained smears were read microscopically and reported as positive for AFBs if any definite red bacilli (singly or in tiny groups, with rods that are either straight or slightly bent or beaded) were seen and negative if no AFBs seen on a blue-green back ground. The results were compared to the gold standard method “GeneXpert” to ascertain results that are real positive, real negative, false positive, and false negative. Sensitivity, specificity, and predictive values were computed from the results after tabulating them as follows [6] (Equation 2);

The test whose calculated value for sensitivity was high was considered to be more sensitive than the other. On addition, the test whose calculated value for specificity was high was considered to be more specific than the other. The test whose Positive predictive value was higher than the other indicated that the test correctly identified the disease. Alternatively, the test whose Negative predictive value was higher than the other indicated that the test identified correctly those without the disease.
Assessing how clinical, sociodemographic, and sample characteristics affect concentrated sputum ZN positivity
The questionnaire was used to gather information on the sociodemographic and clinical aspects like Age, Sex, gender, occupation, locality, smoking status, alcohol consumption status etc. of the patients (Appendix 2). The nature of the sputum samples was recorded i.e. whether; purulent, muco-purulent, mucoid, muco-salivary, and blood stained. The results of positive smears were tabulated and the odds ratios were calculated to determine the effect of the factors on concentrated ZN positivity.
Data analysis and presentation
Determining Sensitivity and specificity of NaOCl concentration and direct ZN methods
The results of this objective were summarized in form of proportions (sensitivity, specificity and predictive values). Data was analyzed by 2X2 contingency table using SPSS software, version 19 at 5% level of significance.
Assessing how clinical, sociodemographic, and sample characteristics affect concentrated sputum ZN positivity
Prediction of ZN Positivity was assessed using Logistic regression analysis basing on prevalence of TB in the study area (Appendix 3: Logistic regression). Interacting effects were assessed using log likelihoods (Chunk test) and non-interacting variables that moved out of model were assessed for confounding effects at 5% level of significance. The final model was written in tabular form showing measure of effect at 95% confidence interval and p.
Quality control
Results for all samples using either method were compared to the gold standard (reference test results) “GeneXpert” which almost has the same sensitivity (92.2%) and specificity (99%) [7] as culture method whose sensitivity is about (81%) and specificity of (98.5%) [8]. This helped in identifying true positive, true negative, false positive and false negative results to assess the statistical findings of direct ZN and NaOCl concentration techniques.
Slides made using both techniques were assessed using the RNTCP guidelines after being individually examined by another skilled Laboratory Technologist using bright field microscopy to eliminate observer bias (Appendix 1).
The smears were stained along with known positive and negative slides to make sure that the stains and microscopic examination were satisfactory.
All stains were filtered daily and put in clean staining containers to avoid any contamination that can lead to artifacts formation.
The smear was not touched with the end of the oil dispenser because this could transfer AFBs from one preparation to another.
Ethical considerations
The research proposal was sent to Mulago Hospital’s research and ethics committee (REC) for review after being approved by Makerere University’s, College of Veterinary Medicine, Animal Resources and Biosecurity, School of Biomedical Laboratory Technology (MREC: 915). A letter of introduction was given to the investigator that introduced him to the head of the laboratory section of the TB unit so that he conducts research there. In order to maintain the highest level of confidentiality, data was coded to avoid patient names.
3. Results
The sensitivity and specificity of NaOCl concentration and direct ZN techniques
This study involved 114 patients in all, with 58 men (50.9%) and 56 women (49.1%). Overall, 120 sputum samples from the 120 patients were obtained; however, 6 samples from 6 individuals were eliminated from the study because they did not match the requirements, leaving 114 samples overall. Ziehl-Nelseen staining findings from the two approaches and GeneXpert were compared. Of the total 114 samples, 82 (71.9%) samples were positive by GeneXpert test (gold standard), whereas by NaOCl Concentration method 79 samples (69.3%) were found positive. However, with direct Ziehl-Nelseen method, 65 samples (57.0%) were found positive, thus a difference of 14 samples in positivity was noted. When compared to the direct Ziehl-Nelseen method with a 57.0% case detection rate, these 14 additional cases diagnosed represented an increase to 69.3%. The rise of 12.3% is very significant when compared to the direct Ziehl-Nelseen method with P<0.005 (χ2=95.38).
Laboratory findings show that there were 65 true positive samples and 16 false negative samples, 3 false positives and 30 true negatives (Table 1). Findings of analysis show that the sensitivity of direct ZN was 80.2% and the specificity of 90.9%. Furthermore, the positive predictive value was 0.96 and the negative predictive value was 0.65. The technique was found to be 83.3% efficient in detecting TB.
A comparison of the direct sputum smear ZN method and sodium hypochlorite concentration method.
Laboratory findings show that there were 79 true positives samples, 2 false negatives, 31 true negatives and 2 false positives (Table 2). Findings of the analysis show that the sensitivity was 97.5% and specificity of 93.9%. Additionally, both the positive and negative predictive values were 0.98 and 0.94 respectively. The technique was found to be 96.5% efficient in detecting AFB and the chi-square distribution showed χ2=95.382 with p<0.05 at α=0.05and df=1.
The Clinical, Socio-demographic, and Sample characteristics’ effects on ZN positivity
The effect of clinical, socio-demographic factors and sample appearance on ZN positivity in this study was not statistically significant. The results from logistic regression indicate that the independent variables do not statistically influence an individual’s TB status (P=0.796) and contribute a paltry 10.6% of the variation in the TB status of a respondent (Table in Appendix 3). A look at each of the variables indicates that there are no statistically significant.
4. Discussion
Currently, direct sputum microscopy is the least expensive and most practical way to diagnose pulmonary tuberculosis in national programs, especially in low-income nations like Uganda. AFB may be found in sputum quickly, cheaply, and with great specificity using direct sputum microscopy. The depressingly low sensitivity of this method is a major drawback. The high incidence of pulmonary tuberculosis with a smear negative result frequently reflect the limited sensitivity [3].
The NaOCl concentration method has a special advantage in overburdened control programmes, where the laboratory workers, because of the large workload, can’t endure to spend the required 5 to 10 minutes in the examination of one slide. Careful preparation and examination of the smear made directly from the sputum gives a sensitivity of round 55% compared with culture, whereas the sensitivity of NaOCl method is around 70%. This shows a difference of 15%. The results in this study show that concentrating sputum with sodium hypochlorite followed by centrifugation had sensitivity of 97.5% and specificity of 93.9% compared to that of direct sputum smear with sensitivity of 80.2% and specificity of 90.9%. Almost a similar difference of 17.3% in sensitivity was obtained with NaOCl concentration method compared to direct sputum smear in this study. This is in agreement with a study [9] which found that bleach digestion improved sensitivity of ZN method from 30.8% to 60.2% in studies done in Ethiopia and India. The increased sensitivity of the NaOCl concentration method of 97.5% is likely attributed to the significantly higher density of bacilli per microscopic field obtained in this method and the reduction of the debris giving a clear field of view as observed in one of the previous studies [4].
It is a prevalent misconception that mycobacteria stay buoyant during centrifugation because of their lipid coat. Contrary to popular opinion, the bleach method has been successful in enabling bacilli to deposit at the bottom of the test tube following centrifugation. According to Annam et al. [10], this better recovery is likely brought about by changes in the surface characteristics of the bacilli (such :as char:ge and hydrophobicity), as well as denaturation of the material, which causes flocculation and subsequently increases the pace at which the AFB sediments. Both observers noted that the fields were clean and had less detritus in the smears made following the sodium hypochlorite treatment. They also noted a notable rise in the average number of AFB seen per field, making the observation less taxing for the observers.
The high sensitivity (80.2%) of direct sputum smear was due to high prevalence of TB in the study population conducted in this setting. This was because TB ward is a referral center for other neighboring facilities hence high prevalence.
NaOCl concentration technique resulted in a 12.3% rise in sputum smear positive overall in this study. Tanzanian researchers [11] reported that using this concentration method as opposed to the direct ZN method, there was an increase in smear positivity by 15.6%. Additionally, one of the previous studies [3] reported a 7.11% increase in positivity. Utilizing sodium hypochlorite as a pre-treatment prior to concentration does not require a lot of labor and can be done by the same technical team with the addition of a centrifuge machine. Only samples that test negative using the standard ZN method can be retested using the sodium hypochlorite concentration method, if not all of them. This can be completed the same day because only a half-hour of preparation is needed. For all patients with negative sputum smear results, results can be provided with a delay of no longer than 24 hours. A significant increase in positivity of 12.3% (from 57.0% to 69.3%) in the new case detection in this study clearly demonstrates that higher sensitivity makes up for a 24-hour reporting delay.
NaOCl has the benefit of lowering the possibility of laboratory infection in addition to being a strong disinfectant. This method is suitable for controlling outbreaks, as NaOCl is inexpensively and widely accessible as household bleach. The time required for the evaluation of the slides is decreased when samples are prepared using the NaOCl concentration method. The workload at TB laboratories might be lessened by this strategy. Though there was a significant increase in sensitivity with NaOCl concentration method, the specificity of NaOCl concentration method and direct sputum smear was relatively the same 93.9% and 90.9% respectively. However, on the same note NaOCl concentration method was found to be more efficient in detecting AFBs than direct sputum smear with 96.5% and 83.3% efficiency respectively. The values obtained from concentration of bacilli by NaOCl concentration method by centrifugation are statically significant (X2 =95.38, P<0.05 at α 0.05 and df=1). The findings of this study demonstrate a considerable increase in the sensitivity of the Ziehl-Nelseen method following the liquefaction of sputum samples with NaOCl and centrifugation. Thus, it was discovered that the concentration method was very sensitive.
There was no statistically significant relationship between the clinical, sociodemographic, sample characteristics and ZN positivity in this investigation. The results from logistic regression indicate that the independent variables do not statistically influence an individual’s TB status (P=0.796) and contribute a paltry 10.6% of the variation in the TB status of a respondent (Appendix 3: Logistic regression). A look at each of the variables indicates that there are no statistically significant. This implies that none of the variables relate with the TB status. It is well known that smoking and drinking contribute to the development of active TB, but in this study, smoking and drinking were not linked to active TB. This finding is likely as result of a social desirability bias in which smokers and drinkers downplayed their use of these substances. This matched the findings of a research by Davies and others [8]. In their study, urban inhabitants had a greater prevalence of PTB with a positive smear (65.4%) than rural residents. This result was in line with research conducted in India, where a researcher [12] reported a prevalence of smear-positive TB of 69.2%. The cramped living circumstances in urban areas may be one of the contributing factors. Additionally, living with a TB patient was one of the most significant predictors of PTB with a positive smear, which may be related to the possibility that increased PTB transmission could result from frequent contact with TB patients in a household. However, the results of this study indicated that there was no statistically significant correlation between ZN positivity and living with a TB patient. Age, sex, educational attainment, employment position, marital status, and sample appearance had no statistically significant correlation with ZN positive (P>0.05).
5. Conclusions
NaOCl concentration method had higher sensitivity than direct sputum smear with 97.5% and 80.2% respectively. Following treatment with 5% sodium hypochlorite solution, increased microscopy sensitivity is most likely caused by concentration, which increases the number of bacilli per field and creates clean fields with less debris.
The specificities were almost the same with NaOCl method at 93.9% and direct sputum smear with 90.9%.
Direct sputum smear showed a positive predictive value of 0.95 and a negative predictive value of 0.65, while the NaOCl concentration approach had a positive predictive value of 0.98 and a negative predictive value of 0.94.
The efficiency of NaOCl concentration method was 96.5% while that of direct sputum smear was 83.3%. This indicated that NaOCl concentration method was more efficient in detecting AFBs than direct sputum smear. Clinical, sociodemographic, and sample characteristics in this study had no statistically significant impact on ZN positive.
Recommendations
According to the findings of this study, the NaOCl concentration approach should be used for an accurate diagnosis of pulmonary tuberculosis. To support this finding, the impact of clinical, sociodemographic, and sample appearance should be re-evaluated with a larger sample size and in field settings.
Ethical Considerations
Compliance with ethical guidelines
Ethics Approval was obtained from Mulago Hospital’s research and ethics committee (REC) (MREC: 915).
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
Conceptualization, Methodology, Validation, Writing-Review &Editing: All authors; Software, Formal Analysis, Investigation, Resources, Data Curation, Writing-Original Draft Preparation: Laban Habokwesiga; Supervision: Musisi Lubowa Nathan.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgements
Many thanks to my supervisor, Mr. Musisi Lubowa Nathan, for his unwavering advice and assistance throughout my time at the institution. Authors whose work has been consulted are highly acknowledged. Finally, thanks to the REC Mulago National Referral Hospital for allowing me to conduct this study.
Tuberculosis remains a major public health problem, with an estimated 2 billion people being infected with Tubercle bacilli worldwide despite the fact that the causative organism was discovered more than 100 years ago [1]. In 2013, 9 million people fell ill with Tuberculosis (TB) and 1.5 million died from the disease. According to the World Health Organization (WHO) [2], low- and middle-income countries account for almost 95% of TB mortality. South-East Asia and the Western Pacific region saw the most new cases of TB, making up 56% of all new cases worldwide. With 280 new cases for every 100,000 people, Africa had the highest rate of new infections in 2013. In Uganda, tuberculosis prevalence rate was 0.2% [2]. However, between 2000 and 2003, TB diagnosis and treatment are thought to have saved an estimated 37 million lives [2]. This therefore shows that if additional and cheaper sensitive diagnostic methods like Concentration methods are put in place for TB early diagnosis, then Tuberculosis disease would easily be reduced to a low rate.
Diagnosis of Mycobacterium tuberculosis depends on examining the sputum for bacteria. The most effective way to find tubercle bacilli is through Mycobacterium culture, however it is incredibly time-consuming and needs extra safety precautions in laboratories. Serological methods lack sensitivity and specificity, making them ineffective in control programs. The nucleic acid amplification techniques are the most promising new methods for diagnosing TB quickly, but the technology is not suitable for control programs in developing nations [1]. In addition, fluorescent microscopy with Auramine-O or Rhodamine staining has been done but is not easily implemented in district health centers as compared to direct Ziehl Nelseen (ZN) microscopy which in turn has got shortcomings like decreased sensitivity.
HIV/TB co-infection makes sputum microscopy less sensitive [3]. The sodium hypochlorite concentration method, one of the safest concentration methods for boosting the sensitivity of direct microscopy for the detection of AFB, can be used to increase the sensitivity of sputum microscopy. The sensitivity of direct ZN microscopy can be greatly increased by sputum liquefaction with sodium hypochlorite (NaOCl/Jik) and concentration through centrifugation [4].
Related previous study findings [1] conducted in BLDEU’s Shri B.M.Patil Medical College, Hospital and Research Centre, India show that there has been a significant increase in sensitivity when sputum is concentrated with 5% sodium hypochlorite. Results show that out of 255 sputum samples analyzed, 25 sputum samples were positive for AFBs by direct smear microscopy while 84 were positive for AFBs in concentrated sputum samples. The ZN sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were 29%, 99%, 96% and 74% respectively with a 95% confidence interval, with use of 5% NaOCl method. Concentrating sputum with 5% NaOCl may improve ZN sensitivity hence improving diagnosis.
Diagnosis of Mycobacterium Tuberculosis remains a challenge in District Health Centers and Clinics in Uganda. Direct Ziehl-Nelseen staining method on sputum samples is the conventional Laboratory method for diagnosis of Tuberculosis. However, there are higher chances of missing TB positive cases due to insufficient sensitivity. This would eventually lead to TB transmission and hence mortality. There is a need of alternative ways of improving sensitivity of sputum microscopy. Therefore, the aim of this study was to find out whether use of Sodium Hypochlorite to concentrate sputum improves sensitivity compared to direct ZN microscopy. The general objective was to compare operating characteristics of NaOCl concentration method and direct Ziehl-Nelseen method on sputum samples for diagnosis of Tuberculosis. The specific objectives were: 1) To determine sensitivity and specificity of ZN on concentrated and unconcentrated sputum samples among TB presumptive patients attending Mulago Hospital. 2. To assess how clinical, sociodemographic, and sample characteristics affect concentrated sputum ZN positivity.
2. Materials and Methods
Study design
This was a cross-sectional study of the comparison of Sodium hypochlorite concentrated and direct Ziehl-Nelseen stained sputum smears processed in the period ranging from January-March 2016.
Study area
The study was conducted in TB ward laboratory, Mulago National Referral Hospital. Mulago Hospital is located three kilometers from Kampala city, the Ugandan capital city.
Target population
All adult TB suspect patients visiting the TB ward at Mulago Hospital in Kampala, Uganda made up the target group.
Sample
Adult patients attending TB ward in Mulago Hospital, Kampala, Uganda between January and March 2016. Early morning sputum samples from 114 patients attending Mulago Hospital TB ward from January to March 2016 of adult TB Presumptive patients of either sex, having a cough for three weeks or more, stayingwith people who have tested positive on a sputum smear regardless of how long they have had a cough, collected in well labeled and clean containers recommended for sputum sample collection with complete laboratory request forms and signed consent forms were received, analyzed and included in the study. Sputum samples without complete filled laboratory request forms and signed consent forms plus those collected in wrong containers were excluded from the study.
Sample size determination (Equation 1):
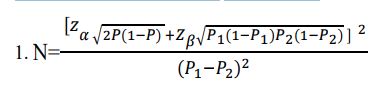
The sample size was determined according KP Suresh & S Chandrashekara’s formular [5].
Where, P1= Sensitivity of NaOCl,
P2= Sensitivity of direct ZN and P=
P1 =60.2% P2 =30.8% P=91% , When expressed as a proportion;
P1=0.602 P2=0.308 P=0.455
Zα is 1.96 and Zβ is 0.84 from the statistical tables. Substituting in the formular, N=49.75.
Minimum sample size (N) is 50 samples. However, for the purposes of increasing statistical power, a sample size of 114 samples was considered in this study.
Data collection methods
The clinical, socio-demographic data, ward, identification number, date, macroscopy and microscopy results of the patients were recorded. The questionnaire was used during collection of such information.
Laboratory procedures
Early morning sputum samples were taken in a sterile, leak-proof labelled container with a wide aperture by patients soon after they wake up and before any mouth wash was used. The patient was requested to cough deeply to produce a sputum specimen.
Two sets of sputum samples were collected and smears were prepared from each sample. The muco-purulent portion of the sputum was placed on a fresh, spotless, and grease free glass labeled slide for the first set, and it was then left to air dry. Dried smears were heat fixed and stained by ZN staining method. During this staining method, strong Carbolfuchsin stain was poured on the heat fixed slides on a staining rack and then heat was applied until vapor came. Slides with the stain were exposed for five minutes. After that, the slide was washed with fresh water and decolored for five minutes using 25% sulfuric acid. This was followed by washing and then counter stained with methylene blue stain for 2 minutes. Finally, the slides were washed and air dried ready for examination.
For the second sample, 1-2 ml of sputum was placed in disposable falcon tubes with a screw lid. The test tube was filled with an equal volume of 5% sodium hypochlorite and left at room temperature for 30 minutes. The test tube was shaken intermittently and about 8 ml of distilled water was added to the test tube. The test tube was then centrifuged at 3000 g for 15 minutes. After carefully discarding the supernatant, the sediments were used to make smears. Heat was used to fix the smears, and the ZN staining procedure was used to stain them similarly (Appendix:1).
Determining the sensitivity and specificity of NaOCl concentration and direct ZN methods
Dried ZN-stained smears were read microscopically and reported as positive for AFBs if any definite red bacilli (singly or in tiny groups, with rods that are either straight or slightly bent or beaded) were seen and negative if no AFBs seen on a blue-green back ground. The results were compared to the gold standard method “GeneXpert” to ascertain results that are real positive, real negative, false positive, and false negative. Sensitivity, specificity, and predictive values were computed from the results after tabulating them as follows [6] (Equation 2);

The test whose calculated value for sensitivity was high was considered to be more sensitive than the other. On addition, the test whose calculated value for specificity was high was considered to be more specific than the other. The test whose Positive predictive value was higher than the other indicated that the test correctly identified the disease. Alternatively, the test whose Negative predictive value was higher than the other indicated that the test identified correctly those without the disease.
Assessing how clinical, sociodemographic, and sample characteristics affect concentrated sputum ZN positivity
The questionnaire was used to gather information on the sociodemographic and clinical aspects like Age, Sex, gender, occupation, locality, smoking status, alcohol consumption status etc. of the patients (Appendix 2). The nature of the sputum samples was recorded i.e. whether; purulent, muco-purulent, mucoid, muco-salivary, and blood stained. The results of positive smears were tabulated and the odds ratios were calculated to determine the effect of the factors on concentrated ZN positivity.
Data analysis and presentation
Determining Sensitivity and specificity of NaOCl concentration and direct ZN methods
The results of this objective were summarized in form of proportions (sensitivity, specificity and predictive values). Data was analyzed by 2X2 contingency table using SPSS software, version 19 at 5% level of significance.
Assessing how clinical, sociodemographic, and sample characteristics affect concentrated sputum ZN positivity
Prediction of ZN Positivity was assessed using Logistic regression analysis basing on prevalence of TB in the study area (Appendix 3: Logistic regression). Interacting effects were assessed using log likelihoods (Chunk test) and non-interacting variables that moved out of model were assessed for confounding effects at 5% level of significance. The final model was written in tabular form showing measure of effect at 95% confidence interval and p.
Quality control
Results for all samples using either method were compared to the gold standard (reference test results) “GeneXpert” which almost has the same sensitivity (92.2%) and specificity (99%) [7] as culture method whose sensitivity is about (81%) and specificity of (98.5%) [8]. This helped in identifying true positive, true negative, false positive and false negative results to assess the statistical findings of direct ZN and NaOCl concentration techniques.
Slides made using both techniques were assessed using the RNTCP guidelines after being individually examined by another skilled Laboratory Technologist using bright field microscopy to eliminate observer bias (Appendix 1).
The smears were stained along with known positive and negative slides to make sure that the stains and microscopic examination were satisfactory.
All stains were filtered daily and put in clean staining containers to avoid any contamination that can lead to artifacts formation.
The smear was not touched with the end of the oil dispenser because this could transfer AFBs from one preparation to another.
Ethical considerations
The research proposal was sent to Mulago Hospital’s research and ethics committee (REC) for review after being approved by Makerere University’s, College of Veterinary Medicine, Animal Resources and Biosecurity, School of Biomedical Laboratory Technology (MREC: 915). A letter of introduction was given to the investigator that introduced him to the head of the laboratory section of the TB unit so that he conducts research there. In order to maintain the highest level of confidentiality, data was coded to avoid patient names.
3. Results
The sensitivity and specificity of NaOCl concentration and direct ZN techniques
This study involved 114 patients in all, with 58 men (50.9%) and 56 women (49.1%). Overall, 120 sputum samples from the 120 patients were obtained; however, 6 samples from 6 individuals were eliminated from the study because they did not match the requirements, leaving 114 samples overall. Ziehl-Nelseen staining findings from the two approaches and GeneXpert were compared. Of the total 114 samples, 82 (71.9%) samples were positive by GeneXpert test (gold standard), whereas by NaOCl Concentration method 79 samples (69.3%) were found positive. However, with direct Ziehl-Nelseen method, 65 samples (57.0%) were found positive, thus a difference of 14 samples in positivity was noted. When compared to the direct Ziehl-Nelseen method with a 57.0% case detection rate, these 14 additional cases diagnosed represented an increase to 69.3%. The rise of 12.3% is very significant when compared to the direct Ziehl-Nelseen method with P<0.005 (χ2=95.38).
Laboratory findings show that there were 65 true positive samples and 16 false negative samples, 3 false positives and 30 true negatives (Table 1). Findings of analysis show that the sensitivity of direct ZN was 80.2% and the specificity of 90.9%. Furthermore, the positive predictive value was 0.96 and the negative predictive value was 0.65. The technique was found to be 83.3% efficient in detecting TB.
A comparison of the direct sputum smear ZN method and sodium hypochlorite concentration method.
Laboratory findings show that there were 79 true positives samples, 2 false negatives, 31 true negatives and 2 false positives (Table 2). Findings of the analysis show that the sensitivity was 97.5% and specificity of 93.9%. Additionally, both the positive and negative predictive values were 0.98 and 0.94 respectively. The technique was found to be 96.5% efficient in detecting AFB and the chi-square distribution showed χ2=95.382 with p<0.05 at α=0.05and df=1.
The Clinical, Socio-demographic, and Sample characteristics’ effects on ZN positivity
The effect of clinical, socio-demographic factors and sample appearance on ZN positivity in this study was not statistically significant. The results from logistic regression indicate that the independent variables do not statistically influence an individual’s TB status (P=0.796) and contribute a paltry 10.6% of the variation in the TB status of a respondent (Table in Appendix 3). A look at each of the variables indicates that there are no statistically significant.
4. Discussion
Currently, direct sputum microscopy is the least expensive and most practical way to diagnose pulmonary tuberculosis in national programs, especially in low-income nations like Uganda. AFB may be found in sputum quickly, cheaply, and with great specificity using direct sputum microscopy. The depressingly low sensitivity of this method is a major drawback. The high incidence of pulmonary tuberculosis with a smear negative result frequently reflect the limited sensitivity [3].
The NaOCl concentration method has a special advantage in overburdened control programmes, where the laboratory workers, because of the large workload, can’t endure to spend the required 5 to 10 minutes in the examination of one slide. Careful preparation and examination of the smear made directly from the sputum gives a sensitivity of round 55% compared with culture, whereas the sensitivity of NaOCl method is around 70%. This shows a difference of 15%. The results in this study show that concentrating sputum with sodium hypochlorite followed by centrifugation had sensitivity of 97.5% and specificity of 93.9% compared to that of direct sputum smear with sensitivity of 80.2% and specificity of 90.9%. Almost a similar difference of 17.3% in sensitivity was obtained with NaOCl concentration method compared to direct sputum smear in this study. This is in agreement with a study [9] which found that bleach digestion improved sensitivity of ZN method from 30.8% to 60.2% in studies done in Ethiopia and India. The increased sensitivity of the NaOCl concentration method of 97.5% is likely attributed to the significantly higher density of bacilli per microscopic field obtained in this method and the reduction of the debris giving a clear field of view as observed in one of the previous studies [4].
It is a prevalent misconception that mycobacteria stay buoyant during centrifugation because of their lipid coat. Contrary to popular opinion, the bleach method has been successful in enabling bacilli to deposit at the bottom of the test tube following centrifugation. According to Annam et al. [10], this better recovery is likely brought about by changes in the surface characteristics of the bacilli (such :as char:ge and hydrophobicity), as well as denaturation of the material, which causes flocculation and subsequently increases the pace at which the AFB sediments. Both observers noted that the fields were clean and had less detritus in the smears made following the sodium hypochlorite treatment. They also noted a notable rise in the average number of AFB seen per field, making the observation less taxing for the observers.
The high sensitivity (80.2%) of direct sputum smear was due to high prevalence of TB in the study population conducted in this setting. This was because TB ward is a referral center for other neighboring facilities hence high prevalence.
NaOCl concentration technique resulted in a 12.3% rise in sputum smear positive overall in this study. Tanzanian researchers [11] reported that using this concentration method as opposed to the direct ZN method, there was an increase in smear positivity by 15.6%. Additionally, one of the previous studies [3] reported a 7.11% increase in positivity. Utilizing sodium hypochlorite as a pre-treatment prior to concentration does not require a lot of labor and can be done by the same technical team with the addition of a centrifuge machine. Only samples that test negative using the standard ZN method can be retested using the sodium hypochlorite concentration method, if not all of them. This can be completed the same day because only a half-hour of preparation is needed. For all patients with negative sputum smear results, results can be provided with a delay of no longer than 24 hours. A significant increase in positivity of 12.3% (from 57.0% to 69.3%) in the new case detection in this study clearly demonstrates that higher sensitivity makes up for a 24-hour reporting delay.
NaOCl has the benefit of lowering the possibility of laboratory infection in addition to being a strong disinfectant. This method is suitable for controlling outbreaks, as NaOCl is inexpensively and widely accessible as household bleach. The time required for the evaluation of the slides is decreased when samples are prepared using the NaOCl concentration method. The workload at TB laboratories might be lessened by this strategy. Though there was a significant increase in sensitivity with NaOCl concentration method, the specificity of NaOCl concentration method and direct sputum smear was relatively the same 93.9% and 90.9% respectively. However, on the same note NaOCl concentration method was found to be more efficient in detecting AFBs than direct sputum smear with 96.5% and 83.3% efficiency respectively. The values obtained from concentration of bacilli by NaOCl concentration method by centrifugation are statically significant (X2 =95.38, P<0.05 at α 0.05 and df=1). The findings of this study demonstrate a considerable increase in the sensitivity of the Ziehl-Nelseen method following the liquefaction of sputum samples with NaOCl and centrifugation. Thus, it was discovered that the concentration method was very sensitive.
There was no statistically significant relationship between the clinical, sociodemographic, sample characteristics and ZN positivity in this investigation. The results from logistic regression indicate that the independent variables do not statistically influence an individual’s TB status (P=0.796) and contribute a paltry 10.6% of the variation in the TB status of a respondent (Appendix 3: Logistic regression). A look at each of the variables indicates that there are no statistically significant. This implies that none of the variables relate with the TB status. It is well known that smoking and drinking contribute to the development of active TB, but in this study, smoking and drinking were not linked to active TB. This finding is likely as result of a social desirability bias in which smokers and drinkers downplayed their use of these substances. This matched the findings of a research by Davies and others [8]. In their study, urban inhabitants had a greater prevalence of PTB with a positive smear (65.4%) than rural residents. This result was in line with research conducted in India, where a researcher [12] reported a prevalence of smear-positive TB of 69.2%. The cramped living circumstances in urban areas may be one of the contributing factors. Additionally, living with a TB patient was one of the most significant predictors of PTB with a positive smear, which may be related to the possibility that increased PTB transmission could result from frequent contact with TB patients in a household. However, the results of this study indicated that there was no statistically significant correlation between ZN positivity and living with a TB patient. Age, sex, educational attainment, employment position, marital status, and sample appearance had no statistically significant correlation with ZN positive (P>0.05).
5. Conclusions
NaOCl concentration method had higher sensitivity than direct sputum smear with 97.5% and 80.2% respectively. Following treatment with 5% sodium hypochlorite solution, increased microscopy sensitivity is most likely caused by concentration, which increases the number of bacilli per field and creates clean fields with less debris.
The specificities were almost the same with NaOCl method at 93.9% and direct sputum smear with 90.9%.
Direct sputum smear showed a positive predictive value of 0.95 and a negative predictive value of 0.65, while the NaOCl concentration approach had a positive predictive value of 0.98 and a negative predictive value of 0.94.
The efficiency of NaOCl concentration method was 96.5% while that of direct sputum smear was 83.3%. This indicated that NaOCl concentration method was more efficient in detecting AFBs than direct sputum smear. Clinical, sociodemographic, and sample characteristics in this study had no statistically significant impact on ZN positive.
Recommendations
According to the findings of this study, the NaOCl concentration approach should be used for an accurate diagnosis of pulmonary tuberculosis. To support this finding, the impact of clinical, sociodemographic, and sample appearance should be re-evaluated with a larger sample size and in field settings.
Ethical Considerations
Compliance with ethical guidelines
Ethics Approval was obtained from Mulago Hospital’s research and ethics committee (REC) (MREC: 915).
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
Conceptualization, Methodology, Validation, Writing-Review &Editing: All authors; Software, Formal Analysis, Investigation, Resources, Data Curation, Writing-Original Draft Preparation: Laban Habokwesiga; Supervision: Musisi Lubowa Nathan.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgements
Many thanks to my supervisor, Mr. Musisi Lubowa Nathan, for his unwavering advice and assistance throughout my time at the institution. Authors whose work has been consulted are highly acknowledged. Finally, thanks to the REC Mulago National Referral Hospital for allowing me to conduct this study.
- Montali RJ, Mikota SK, Cheng LI. Mycobacterium tuberculosis in zoo and wildlife species. Rev Sci Tech. 2001; 20(1):291-303. [DOI:10.20506/rst.20.1.1268] [PMID]
- WHO. Global tuberculosis report 2014. Geneva: World Health Organization; 2014. [Link]
- Kaore NM, Date KP, Thombare VR. Increased sensitivity of sputum microscopy with sodium hypochlorite concentration technique: A practical experience at RNTCP center. Lung India. 2011; 28(1):17-20. [DOI:10.4103/0970-2113.76295] [PMID] [PMCID]
- Chandrasekhar B, Prayaga AK. Utility of concentration method by modified bleach technique for the demonstration of acid-fast bacilli in the diagnosis of tuberculous lymphadenopathy. J Cytol. 2012; 29(3):165-8. [DOI:10.4103/0970-9371.101160] [PMID] [PMCID]
- Suresh K, Chandrashekara S. Sample size estimation and power analysis for clinical research studies. J Hum Reprod Sci. 2012; 5(1):7-13. [PMID] [PMCID]
- Habibzadeh F, Habibzadeh P, Yadollahie M. The apparent prevalence, the true prevalence. Biochem Med. 2022; 32(2):020101. [DOI:10.11613/BM.2022.020101] [PMID] [PMCID]
- Brown M, Varia H, Bassett P, Davidson RN, Wall R, Pasvol G. Prospective study of sputum induction, gastric washing, and bronchoalveolar lavage for the diagnosis of pulmonary tuberculosis in patients who are unable to expectorate. Clin Infect Dis. 2007; 44(11):1415-20. [DOI:10.1086/516782] [PMID]
- Davies PD, Yew WW, Ganguly D, Davidow AL, Reichman LB, Dheda K, et al. Smoking and tuberculosis: The epidemiological association and immunopathogenesis. Trans R Soc Trop Med Hyg. 2006; 100(4):291-8. [DOI:10.1016/j.trstmh.2005.06.034] [PMID]
- Gebre N, Karlsson U, Jönsson G, Macaden R, Wolde A, Assefa A, et al. Improved microscopical diagnosis of pulmonary tuberculosis in developing countries. Trans R Soc Trop Med Hyg. 1995; 89(2):191-3. [DOI:10.1016/0035-9203(95)90491-3] [PMID]
- Annam V, Karigoudar MH, Yelikar BR. Improved microscopical detection of acid-fast bacilli by the modified bleach method in lymphnode aspirates. Indian J Pathol Microbiol. 2009; 52(3):349-52. [DOI:10.4103/0377-4929.54991] [PMID]
- Makunde WH, Makunde RA, Kamugisha LM, Mgema SG, Liwa A. Improved microscopy diagnosis of pulmonary tuberculosis using sodium hypochlorite concentration technique in Tanga, Tanzania. Tanzan Health Res Bull. 2007; 9(2):87-93. [DOI:10.4314/thrb.v9i2.14309] [PMID]
- Stevenson CR, Forouhi NG, Roglic G, Williams BG, Lauer JA, Dye C, et al. Diabetes and tuberculosis: The impact of the diabetes epidemic on tuberculosis incidence. BMC Public Health. 2007; 7:234. [DOI:10.1186/1471-2458-7-234] [PMID] [PMCID]
Article Type: Original Contributions |
Subject:
Public Health
Received: 2023/02/10 | Accepted: 2023/04/29 | Published: 2023/06/26
Received: 2023/02/10 | Accepted: 2023/04/29 | Published: 2023/06/26
Send email to the article author
| Rights and permissions | |
 | This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |









