Volume 10, Issue 1 (1-2025)
CJHR 2025, 10(1): 73-80 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Massahi T, Kiani A, Almasi A, Jaafari J, Fattahi N, Sharafi K. Migration of Phthalates From Polyethylene Terephthalate Bottles into Beverages: A Health Policy Brief. CJHR 2025; 10 (1) :73-80
URL: http://cjhr.gums.ac.ir/article-1-387-en.html
URL: http://cjhr.gums.ac.ir/article-1-387-en.html
1- Student Research Committee, Kermanshah University of Medical Sciences, Kermanshah, Iran.
2- Regenerative Medicine Research Center, Research Institute for Health Technology, Kermanshah University of Medical Sciences, Kermanshah, Iran.
3- Social Development & Health Promotion Research Center, Health Institute, Kermanshah University of Medical Sciences, Kermanshah, Iran.
4- Department of Environmental Health Engineering, Research Center of Health and Environment, Guilan University of Medical Sciences, Rasht, Iran.
5- Research Center for Environmental determinants of Health, Health Institute, Kermanshah University of Medical Sciences, Kermanshah, Iran.
6- Social Development & Health Promotion Research Center, Health Institute, Kermanshah University of Medical Sciences, Kermanshah, Iran. ,kio.sharafi@gmail.com
2- Regenerative Medicine Research Center, Research Institute for Health Technology, Kermanshah University of Medical Sciences, Kermanshah, Iran.
3- Social Development & Health Promotion Research Center, Health Institute, Kermanshah University of Medical Sciences, Kermanshah, Iran.
4- Department of Environmental Health Engineering, Research Center of Health and Environment, Guilan University of Medical Sciences, Rasht, Iran.
5- Research Center for Environmental determinants of Health, Health Institute, Kermanshah University of Medical Sciences, Kermanshah, Iran.
6- Social Development & Health Promotion Research Center, Health Institute, Kermanshah University of Medical Sciences, Kermanshah, Iran. ,
Keywords: Phthalate, Polyethylene terephthalate (PET), Beverages, Bottled water, Health policy, Iran
Full-Text [PDF 511 kb]
(771 Downloads)
| Abstract (HTML) (1092 Views)
Full-Text: (1162 Views)
Introduction
In recent decades, the widespread use of plastics in daily life and the inevitable human interaction with plastic products and their harmful components that alter the human endocrine system have raised concerns. Endocrine-disrupting chemicals (EDCs) are a large group of natural and synthetic substances that interfere with the normal functions of the endocrine system. These chemical compounds include environmental estrogens (xenoestrogens) such as phytoestrogens or artificial chemicals such as alkylphenols and phthalates [1]. These xenoestrogenic compounds are a diverse group that bind to estrogen receptors, mimic estrogenic effects, and may have adverse effects on human health [2, 3].
Phthalates (also called phthalic diesters) are a group of related organic chemicals commonly used as plasticizers in the plastics industry. Polyvinyl chloride (PVC) and other polymers such as rubber and styrene, commonly have plasticizers added to them to make them flexible and elastic. The five most commonly used phthalates in the plastics industry include: Di-(2-ethylhexyl) phthalate (DEHP), dibutyl phthalate (DBP), diisononyl phthalate (DINP), diisodecyl phthalate (DIDP), and benzyl butyl phthalate (BBP) [4]. Because phthalates are not tightly bound to plastic, they can migrate into water or food over time when they come into contact with the plastic container [5]. BBP, DEHP, and DBP are listed as EDCs [6]. Human exposure to phthalates can lead to health problems including breast cancer, reproductive hormonal disorders, obesity, and impaired function of enzymes involved in sexual cell maturation [7, 8].
In Iran, polyethylene terephthalate (PET) bottles are widely used in the production of mineral water and various beverages due to their low cost and easy availability. Most commonly, plastic containers are used as drinking water bottles, and the unfavorable storage conditions of these bottles in factories and distribution warehouses (in direct sunlight) increase the temperature inside the water bottles, which can increase the likelihood of migration of compounds such as phthalates from the bottle wall into the water contained.
This health policy brief identifies the factors influencing phthalate migration and provides recommendations to reduce the associated risks through effective public health policies and practices.
Materials and Methods
To develop this policy brief, we first conducted a comprehensive search of reputable national and international databases, including Google Scholar, ScienceDirect, Scopus, PubMed, Iranian Research Institute for Information Science and Technology (IranDoc), Comprehensive reference for Iranian Scientific and Specialized Journals (Magiran) and ISC (Islamic World Science Citation). Relevant keywords such as “polyethylene terephthalate”, “beverages”, “migration”, “bottle”, “phthalate”, “concentration”, “liquids”, and “storage” were used in the search and combined (and/or) to conduct a comprehensive search. This search included scientific reports and studies on the subject from the last two decades. We then used the key findings from these studies to determine which method of storing PET bottles is best to prevent the formation of phthalate compounds. Finally, by modeling the measures taken in other countries, we have made important recommendations to prevent the migration of phthalates from the walls of bottles into drinks.
Results
Various studies have been carried out around the world to detect phthalates in PET bottled beverages. Table 1 summarizes the phthalate concentrations observed in diverse studies, highlighting their variability based on storage conditions and bottle reuse.
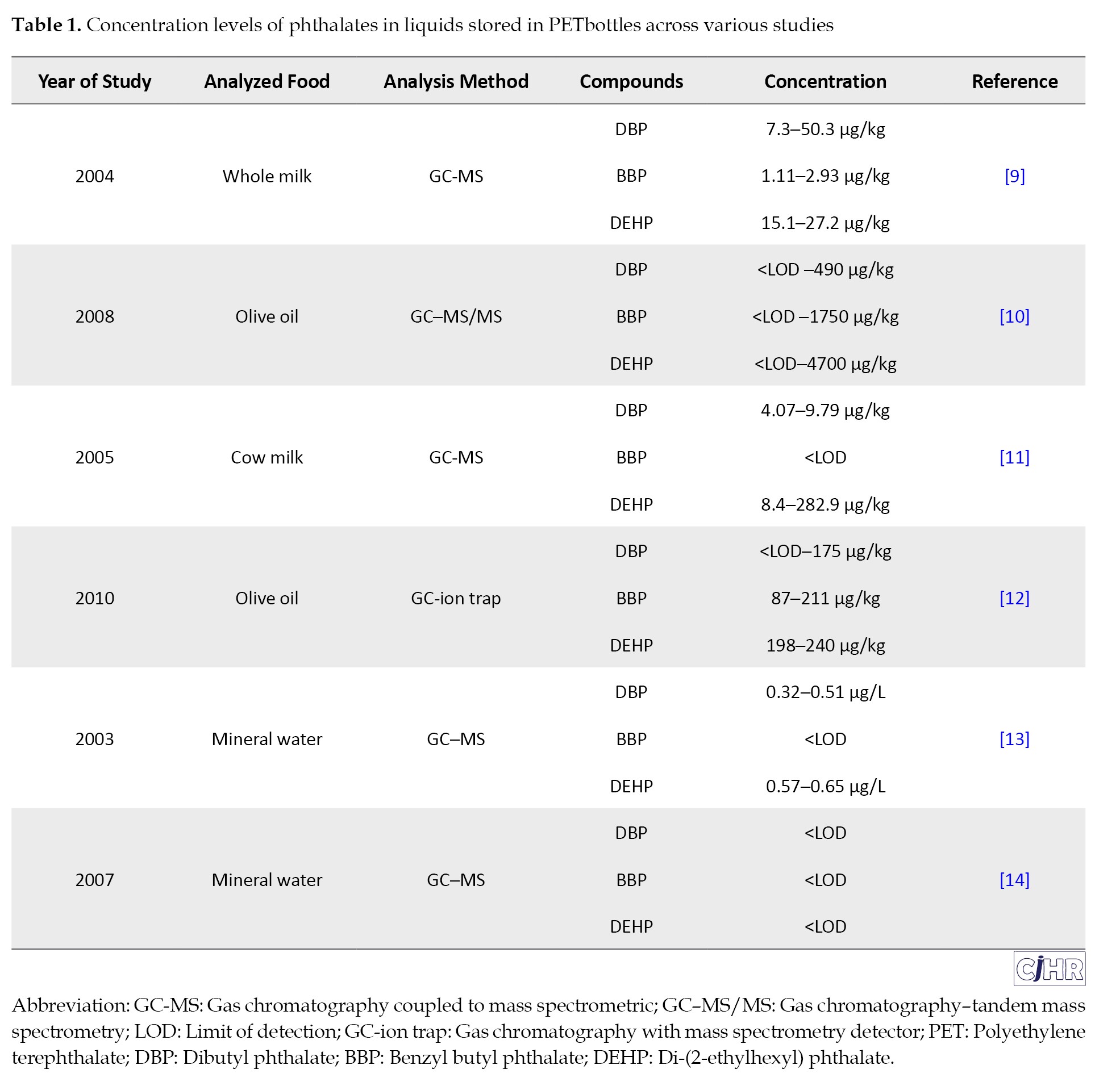
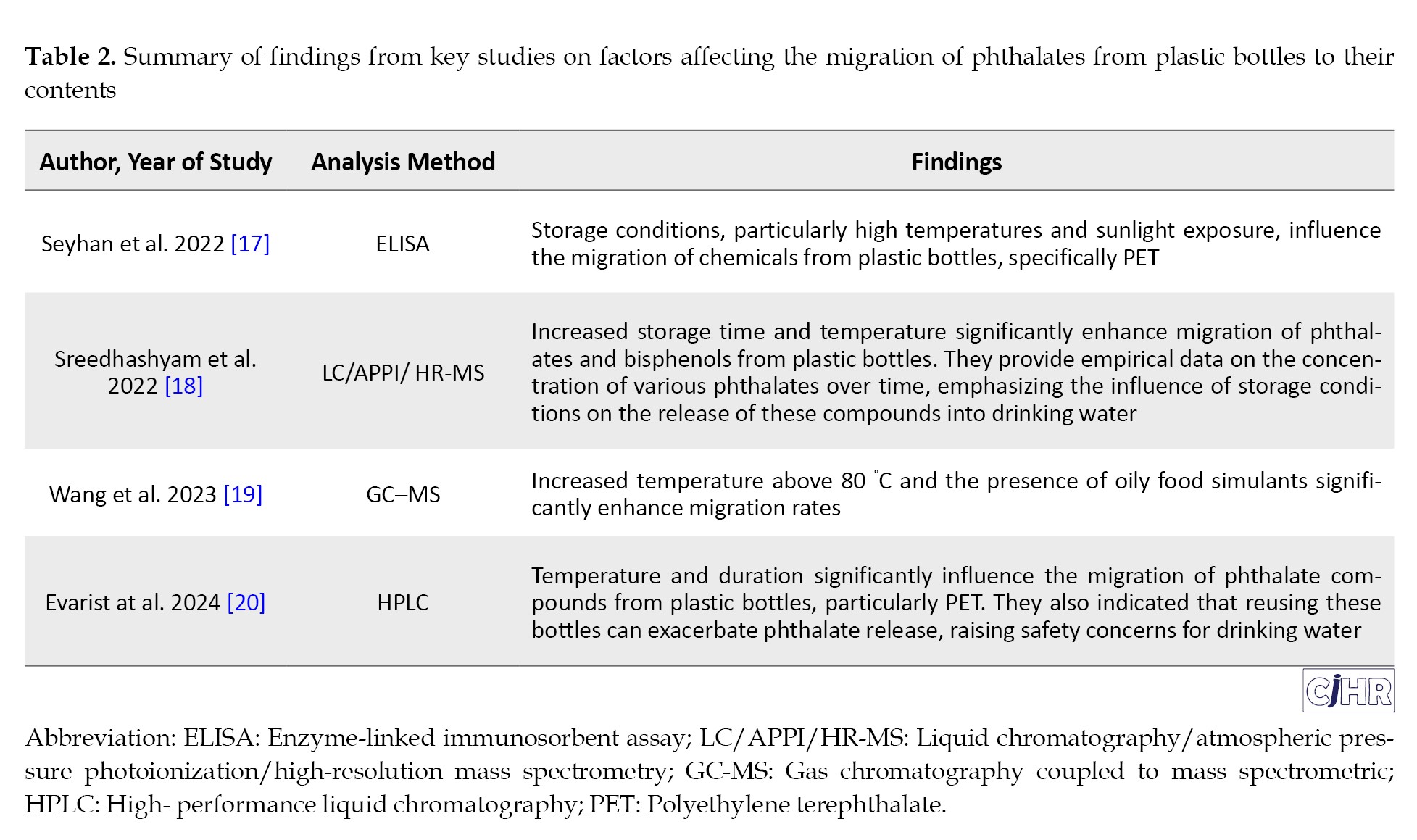
Factors affecting the migration of phthalates from the bottle wall into the beverage have been reported in some studies. In a study conducted by Keresztes et al. (2013) in Hungary, the migration of phthalates from PET bottles into drinking water was investigated. The results showed that the levels of DIBP, DBP, BBP, and DEHP in the water contained in the tested bottles were in the range of >3-200, >6.6-800, >6-100, and >16-170 ng/L, respectively. Among the phthalates studied, DEHP was the most prevalent in the samples [7]. A study by Moreira et al. (2014) in Brazil examined DBP migration from plastic materials to food during the microwaving process. The results showed that the levels of DBP in the food samples were in the range of >0.08-7.5 μg/L, and that increasing the heating time and the frequency of using plastic containers increased the phthalate content in food [15]. A study by Jeddi et al. (2015) investigated the levels of phthalates in bottled water under common storage conditions. The results showed that increasing the storage temperature and duration increased the migration of phthalates from the bottle wall into the water [16]. Table 2 presents a compilation of results from diverse research studies, providing insights into the factors that influence the migration of phthalates.
Discussion
Phthalates are commonly used plasticizers in the plastics industry [4, 6] and are not chemically bound to the polymer matrix of the plastic. This lack of chemical binding makes them prone to migration into foods or beverages that come into contact with plastic containers [5, 21]. The serious adverse health effects of phthalates have garnered significant attention from researchers and health authorities. The migration process of phthalates from PET bottle walls into their contents depends on various factors, including the concentration of phthalates in the packaging material, the acidity and lipid content of the contents that come into contact with the bottles, and the duration of the liquid’s contact with the bottle wall (storage time), the temperature of the environment in which the bottles with liquids are stored, and whether or not they are exposed to sunlight [5, 16, 22-25].
The unique chemical properties of DEHP, DBP and BBP may contribute to their migration behavior into the bottle contents. These compounds exhibit a higher tendency tendency to migrate into food or beverages under various real-world conditions. In addition, the contact time between the PET bottle and its contents can be a significant factor in chemical migration. Prolonged contact at higher temperatures can increase leaching and potentially lead to increased phthalate levels in the stored food or beverage. In addition, the properties of the contents in the bottles, such as pH and the presence of additives, also play a crucial role in the migration of these compounds. These external elements can influence chemical interactions in PET bottles and influence the leaching process of phthalates into stored food or beverages [5, 26, 27]. Xu et al. (2010) reported that the migration rate of phthalates from the walls of bottles specifically designed for cooking oil was higher than that of bottles specifically designed for bottled water. Among the factors studied, storage temperature and duration were found to have the most substantial impact on migration rates [28].
High temperatures can accelerate the degradation of plastic polymers and increase the mobility of chemical additives, resulting in higher phthalate release [21]. This phenomenon is particularly noticeable in scenarios where PET bottles are exposed to high temperatures, such as when stored outdoors in direct sunlight. To assess the safety of PET bottles, it is crucial to understand the extent to which sunlight influences phthalate migration. This issue is particularly relevant in a country like Iran, where observations indicate that some sellers or consumers store excess packaged foods outdoors (usually outside the distribution and sales area) due to limited storage space. Findings from a study by Jeddi et al. (2015) in Iran showed that increasing the storage temperature and duration of mineral water bottles resulted in an increase in the migration of phthalates from the bottle wall into the contained water [16].
It has been proven that increasing temperature and storage time, in addition to phthalates, can also increase the migration of other dangerous compounds from the bottle wall into the liquids contained. Chapa-Martínez et al. (2016) in Mexico reported that among the variables studied (temperature, pH, and storage time), temperature had the greatest influence on the migration rate of antimony from PET bottles into water. The highest migration occurred at a pH of 7, a temperature of 75 °C and a storage period of 5 days [29]. Kazemi et al. (2012) found that increasing temperatures significantly increase the migration rate of bisphenol A (BPA) and nonylphenol from PET bottles into water [30]. In a study by Nam et al. (2010) in South Korea showed that the migration rate of BPA from a polycarbonate baby bottle into water was 0.03 ppb and 0.13 ppb at 40 °C and 95 °C, respectively, when the bottle was new. However, after 6 months of use at the same temperatures, these values increased to 0.18 ppb and 18.47 ppb. Based on the results of this study, it was found that the migration rate of BPA from bottle to water increased rapidly at temperatures above 80 °C, and the migration rate of BPA from bottle to water was 4.9×10-2 ppb at each use [31].
On the other hand, some Iranian consumers, after consuming beverages and liquids from new bottles, reuse them for the same purpose (e.g. drinking water) or for other purposes (e.g. storing vinegar, lemon juice, herbal extracts, pickles, etc.). Therefore, different temperature storage conditions and the type of food stored in the bottles (with pH levels higher or lower than neutral) can affect the release of phthalates [6]. Despite the prevalence of reusing PET bottles in Iran, there is a lack of comprehensive data on how the reuse of these bottles affects the migration of phthalates from PET bottles into beverages or other stored substances. Therefore, conducting research on this topic is necessary.
Based on the findings, we present several recommendations for the proper production, storage and transportation of PET beverage bottles to reduce the migration of phthalates and other harmful compounds into the beverages. During the production process, manufacturers should prioritize the use of high-quality PET materials with minimal phthalate content to reduce the potential for migration.
Temperature control is crucial for storage and transportation. Bottles should be kept in cool environments to slow down the migration of phthalates. Additionally, exposure of beverage-containing bottles to direct sunlight should be avoided, as higher temperatures can accelerate the release of harmful compounds from the bottles. Distributors and retailers should also be trained on appropriate storage methods, emphasizing the importance of keeping bottles away from heat sources and sunlight.
The duration of storage should also be minimized, as longer contact times between the beverage and the bottle walls increase the risk of phthalate migration. Monitoring the inventory of retailers can help ensure that bottles are not stored for extended periods before reaching consumers. Furthermore, reuse of PET bottles should be reduced whenever possible, especially for storing acidic or fatty substances, as these can increase the leaching of phthalates.
Transportation methods should prioritize temperature-controlled vehicles to maintain a consistent cool temperature throughout transit. Packaging and handling methods should be designed to minimize physical stress on bottles, as this can potentially accelerate the release of compounds from the plastic. By implementing these measures, the risk of phthalate migration from PET bottles into beverages can be significantly reduced, ensuring product safety for consumers.
Conclusion
Previous studies indicate that factors such as increased storage temperature, exposure to direct sunlight, prolonged storage duration (contact time between the beverage and the inner wall of the bottle), and the reuse of bottles significantly contribute to the migration of hazardous compounds, including phthalates, from PET bottle walls into the contained beverages.
To address these risks, it is recommended that health authorities and regulatory bodies implement targeted policies and issue guidelines for beverage manufacturers to ensure proper storage conditions for PET bottles. Furthermore, public education and information campaigns are essential to raise awareness among consumers, distributors, and retailers about the correct storage and usage practices for PET bottles. These efforts will help mitigate the release of harmful substances and enhance the safety of bottled beverages.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Ethics Committee of Kermanshah University of Medical Sciences (Code: IR.KUMS.REC.1399.359).
Funding
This study was supported by the Research Council of Kermanshah University of Medical Sciences, Kermanshah, Iran (Grant No.: 990598).
Authors' contributions
All authors contributed equally to the conception and design of the study, data collection and analysis, interception of the results and drafting of the manuscript. Each author approved the final version of the mnuscript for submission.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgements
The authors would like to thank the Research Council of Kermanshah University of Medical Sciences, Kermanshah, Iran for their support.
References
In recent decades, the widespread use of plastics in daily life and the inevitable human interaction with plastic products and their harmful components that alter the human endocrine system have raised concerns. Endocrine-disrupting chemicals (EDCs) are a large group of natural and synthetic substances that interfere with the normal functions of the endocrine system. These chemical compounds include environmental estrogens (xenoestrogens) such as phytoestrogens or artificial chemicals such as alkylphenols and phthalates [1]. These xenoestrogenic compounds are a diverse group that bind to estrogen receptors, mimic estrogenic effects, and may have adverse effects on human health [2, 3].
Phthalates (also called phthalic diesters) are a group of related organic chemicals commonly used as plasticizers in the plastics industry. Polyvinyl chloride (PVC) and other polymers such as rubber and styrene, commonly have plasticizers added to them to make them flexible and elastic. The five most commonly used phthalates in the plastics industry include: Di-(2-ethylhexyl) phthalate (DEHP), dibutyl phthalate (DBP), diisononyl phthalate (DINP), diisodecyl phthalate (DIDP), and benzyl butyl phthalate (BBP) [4]. Because phthalates are not tightly bound to plastic, they can migrate into water or food over time when they come into contact with the plastic container [5]. BBP, DEHP, and DBP are listed as EDCs [6]. Human exposure to phthalates can lead to health problems including breast cancer, reproductive hormonal disorders, obesity, and impaired function of enzymes involved in sexual cell maturation [7, 8].
In Iran, polyethylene terephthalate (PET) bottles are widely used in the production of mineral water and various beverages due to their low cost and easy availability. Most commonly, plastic containers are used as drinking water bottles, and the unfavorable storage conditions of these bottles in factories and distribution warehouses (in direct sunlight) increase the temperature inside the water bottles, which can increase the likelihood of migration of compounds such as phthalates from the bottle wall into the water contained.
This health policy brief identifies the factors influencing phthalate migration and provides recommendations to reduce the associated risks through effective public health policies and practices.
Materials and Methods
To develop this policy brief, we first conducted a comprehensive search of reputable national and international databases, including Google Scholar, ScienceDirect, Scopus, PubMed, Iranian Research Institute for Information Science and Technology (IranDoc), Comprehensive reference for Iranian Scientific and Specialized Journals (Magiran) and ISC (Islamic World Science Citation). Relevant keywords such as “polyethylene terephthalate”, “beverages”, “migration”, “bottle”, “phthalate”, “concentration”, “liquids”, and “storage” were used in the search and combined (and/or) to conduct a comprehensive search. This search included scientific reports and studies on the subject from the last two decades. We then used the key findings from these studies to determine which method of storing PET bottles is best to prevent the formation of phthalate compounds. Finally, by modeling the measures taken in other countries, we have made important recommendations to prevent the migration of phthalates from the walls of bottles into drinks.
Results
Various studies have been carried out around the world to detect phthalates in PET bottled beverages. Table 1 summarizes the phthalate concentrations observed in diverse studies, highlighting their variability based on storage conditions and bottle reuse.

Factors affecting the migration of phthalates from the bottle wall into the beverage have been reported in some studies. In a study conducted by Keresztes et al. (2013) in Hungary, the migration of phthalates from PET bottles into drinking water was investigated. The results showed that the levels of DIBP, DBP, BBP, and DEHP in the water contained in the tested bottles were in the range of >3-200, >6.6-800, >6-100, and >16-170 ng/L, respectively. Among the phthalates studied, DEHP was the most prevalent in the samples [7]. A study by Moreira et al. (2014) in Brazil examined DBP migration from plastic materials to food during the microwaving process. The results showed that the levels of DBP in the food samples were in the range of >0.08-7.5 μg/L, and that increasing the heating time and the frequency of using plastic containers increased the phthalate content in food [15]. A study by Jeddi et al. (2015) investigated the levels of phthalates in bottled water under common storage conditions. The results showed that increasing the storage temperature and duration increased the migration of phthalates from the bottle wall into the water [16]. Table 2 presents a compilation of results from diverse research studies, providing insights into the factors that influence the migration of phthalates.

Discussion
Phthalates are commonly used plasticizers in the plastics industry [4, 6] and are not chemically bound to the polymer matrix of the plastic. This lack of chemical binding makes them prone to migration into foods or beverages that come into contact with plastic containers [5, 21]. The serious adverse health effects of phthalates have garnered significant attention from researchers and health authorities. The migration process of phthalates from PET bottle walls into their contents depends on various factors, including the concentration of phthalates in the packaging material, the acidity and lipid content of the contents that come into contact with the bottles, and the duration of the liquid’s contact with the bottle wall (storage time), the temperature of the environment in which the bottles with liquids are stored, and whether or not they are exposed to sunlight [5, 16, 22-25].
The unique chemical properties of DEHP, DBP and BBP may contribute to their migration behavior into the bottle contents. These compounds exhibit a higher tendency tendency to migrate into food or beverages under various real-world conditions. In addition, the contact time between the PET bottle and its contents can be a significant factor in chemical migration. Prolonged contact at higher temperatures can increase leaching and potentially lead to increased phthalate levels in the stored food or beverage. In addition, the properties of the contents in the bottles, such as pH and the presence of additives, also play a crucial role in the migration of these compounds. These external elements can influence chemical interactions in PET bottles and influence the leaching process of phthalates into stored food or beverages [5, 26, 27]. Xu et al. (2010) reported that the migration rate of phthalates from the walls of bottles specifically designed for cooking oil was higher than that of bottles specifically designed for bottled water. Among the factors studied, storage temperature and duration were found to have the most substantial impact on migration rates [28].
High temperatures can accelerate the degradation of plastic polymers and increase the mobility of chemical additives, resulting in higher phthalate release [21]. This phenomenon is particularly noticeable in scenarios where PET bottles are exposed to high temperatures, such as when stored outdoors in direct sunlight. To assess the safety of PET bottles, it is crucial to understand the extent to which sunlight influences phthalate migration. This issue is particularly relevant in a country like Iran, where observations indicate that some sellers or consumers store excess packaged foods outdoors (usually outside the distribution and sales area) due to limited storage space. Findings from a study by Jeddi et al. (2015) in Iran showed that increasing the storage temperature and duration of mineral water bottles resulted in an increase in the migration of phthalates from the bottle wall into the contained water [16].
It has been proven that increasing temperature and storage time, in addition to phthalates, can also increase the migration of other dangerous compounds from the bottle wall into the liquids contained. Chapa-Martínez et al. (2016) in Mexico reported that among the variables studied (temperature, pH, and storage time), temperature had the greatest influence on the migration rate of antimony from PET bottles into water. The highest migration occurred at a pH of 7, a temperature of 75 °C and a storage period of 5 days [29]. Kazemi et al. (2012) found that increasing temperatures significantly increase the migration rate of bisphenol A (BPA) and nonylphenol from PET bottles into water [30]. In a study by Nam et al. (2010) in South Korea showed that the migration rate of BPA from a polycarbonate baby bottle into water was 0.03 ppb and 0.13 ppb at 40 °C and 95 °C, respectively, when the bottle was new. However, after 6 months of use at the same temperatures, these values increased to 0.18 ppb and 18.47 ppb. Based on the results of this study, it was found that the migration rate of BPA from bottle to water increased rapidly at temperatures above 80 °C, and the migration rate of BPA from bottle to water was 4.9×10-2 ppb at each use [31].
On the other hand, some Iranian consumers, after consuming beverages and liquids from new bottles, reuse them for the same purpose (e.g. drinking water) or for other purposes (e.g. storing vinegar, lemon juice, herbal extracts, pickles, etc.). Therefore, different temperature storage conditions and the type of food stored in the bottles (with pH levels higher or lower than neutral) can affect the release of phthalates [6]. Despite the prevalence of reusing PET bottles in Iran, there is a lack of comprehensive data on how the reuse of these bottles affects the migration of phthalates from PET bottles into beverages or other stored substances. Therefore, conducting research on this topic is necessary.
Based on the findings, we present several recommendations for the proper production, storage and transportation of PET beverage bottles to reduce the migration of phthalates and other harmful compounds into the beverages. During the production process, manufacturers should prioritize the use of high-quality PET materials with minimal phthalate content to reduce the potential for migration.
Temperature control is crucial for storage and transportation. Bottles should be kept in cool environments to slow down the migration of phthalates. Additionally, exposure of beverage-containing bottles to direct sunlight should be avoided, as higher temperatures can accelerate the release of harmful compounds from the bottles. Distributors and retailers should also be trained on appropriate storage methods, emphasizing the importance of keeping bottles away from heat sources and sunlight.
The duration of storage should also be minimized, as longer contact times between the beverage and the bottle walls increase the risk of phthalate migration. Monitoring the inventory of retailers can help ensure that bottles are not stored for extended periods before reaching consumers. Furthermore, reuse of PET bottles should be reduced whenever possible, especially for storing acidic or fatty substances, as these can increase the leaching of phthalates.
Transportation methods should prioritize temperature-controlled vehicles to maintain a consistent cool temperature throughout transit. Packaging and handling methods should be designed to minimize physical stress on bottles, as this can potentially accelerate the release of compounds from the plastic. By implementing these measures, the risk of phthalate migration from PET bottles into beverages can be significantly reduced, ensuring product safety for consumers.
Conclusion
Previous studies indicate that factors such as increased storage temperature, exposure to direct sunlight, prolonged storage duration (contact time between the beverage and the inner wall of the bottle), and the reuse of bottles significantly contribute to the migration of hazardous compounds, including phthalates, from PET bottle walls into the contained beverages.
To address these risks, it is recommended that health authorities and regulatory bodies implement targeted policies and issue guidelines for beverage manufacturers to ensure proper storage conditions for PET bottles. Furthermore, public education and information campaigns are essential to raise awareness among consumers, distributors, and retailers about the correct storage and usage practices for PET bottles. These efforts will help mitigate the release of harmful substances and enhance the safety of bottled beverages.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Ethics Committee of Kermanshah University of Medical Sciences (Code: IR.KUMS.REC.1399.359).
Funding
This study was supported by the Research Council of Kermanshah University of Medical Sciences, Kermanshah, Iran (Grant No.: 990598).
Authors' contributions
All authors contributed equally to the conception and design of the study, data collection and analysis, interception of the results and drafting of the manuscript. Each author approved the final version of the mnuscript for submission.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgements
The authors would like to thank the Research Council of Kermanshah University of Medical Sciences, Kermanshah, Iran for their support.
References
- Yoshihara S, Makishima M, Suzuki N, Ohta S. Metabolic activation of bisphenol A by rat liver S9 fraction. Toxicol Sci. 2001; 62(2):221-7. [DOI:10.1093/toxsci/62.2.221] [PMID]
- Wagner M, Oehlmann J. Endocrine disruptors in bottled mineral water: Total estrogenic burden and migration from plastic bottles. Environ Sci Pollut Res Int. 2009; 16(3):278-86. [DOI:10.1007/s11356-009-0107-7] [PMID]
- Céspedes R, Petrovic M, Raldúa D, Saura U, Piña B, Lacorte S, et al. Integrated procedure for determination of endocrine-disrupting activity in surface waters and sediments by use of the biological technique recombinant yeast assay and chemical analysis by LC-ESI-MS. Anal Bioanal Chem. 2004; 378(3):697-708. [DOI:10.1007/s00216-003-2303-5] [PMID]
- Wormuth M, Scheringer M, Vollenweider M, Hungerbühler K. What are the sources of exposure to eight frequently used phthalic acid esters in Europeans? Risk Anal. 2006; 26(3):803-24. [DOI:10.1111/j.1539-6924.2006.00770.x] [PMID]
- Amiridou D, Voutsa D. Alkylphenols and phthalates in bottled waters. J Hazard Mater. 2011; 185(1):281-6. [DOI:10.1016/j.jhazmat.2010.09.031] [PMID]
- Dickson‐Spillmann M, Siegrist M, Keller C, Wormuth M. Phthalate exposure through food and consumers’ risk perception of chemicals in food. Risk Anal. 2009; 29(8):1170-81. [DOI:10.1111/j.1539-6924.2009.01233.x] [PMID]
- Keresztes S, Tatár E, Czegeny Z, Záray G, Mihucz VG. Study on the leaching of phthalates from polyethylene terephthalate bottles into mineral water. Sci Total Environ. 2013; 458-460:451-8. [DOI:10.1016/j.scitotenv.2013.04.056] [PMID]
- Parmar D, Srivastava SP, Seth PK. Effect of di (2-ethylhexyl) phthalate (DEHP) on spermatogenesis in adult rats. Toxicology. 1986; 42(1):47-55. [DOI:10.1016/0300-483X(86)90091-0] [PMID]
- Casajuana N, Lacorte S. New methodology for the determination of phthalate esters, bisphenol A, bisphenol A diglycidyl ether, and nonylphenol in commercial whole milk samples. J Agric Food Chem. 2004; 52(12):3702-7. [DOI:10.1021/jf040027s] [PMID]
- Cavaliere B, Macchione B, Sindona G, Tagarelli A. Tandem mass spectrometry in food safety assessment: The determination of phthalates in olive oil. J Chromatogr A. 2008; 1205(1-2):137-43. [DOI:10.1016/j.chroma.2008.08.009] [PMID]
- Feng YL, Zhu J, Sensenstein R. Development of a headspace solid-phase microextraction method combined with gas chromatography mass spectrometry for the determination of phthalate esters in cow milk. Anal Chim Acta. 2005; 538(1-2):41-8. [DOI:10.1016/j.aca.2005.02.020]
- Rios JJ, Morales A, Márquez-Ruiz G. Headspace solid-phase microextraction of oil matrices heated at high temperature and phthalate esters determination by gas chromatography multistage mass spectrometry. Talanta. 2010; 80(5):2076-82. [DOI:10.1016/j.talanta.2009.11.008] [PMID]
- Psillakis E, Kalogerakis N. Hollow-fibre liquid-phase microextraction of phthalate esters from water. J Chromatogr A. 2003; 999(1-2):145-53. [DOI:10.1016/S0021-9673(03)00390-X] [PMID]
- Farahani H, Norouzi P, Dinarvand R, Ganjali MR. Development of dispersive liquid-liquid microextraction combined with gas chromatography-mass spectrometry as a simple, rapid and highly sensitive method for the determination of phthalate esters in water samples. J Chromatogr A. 2007; 1172(2):105-12. [DOI:10.1016/j.chroma.2007.10.001] [PMID]
- Moreira MA, André LC, Cardeal ZL. Analysis of phthalate migration to food simulants in plastic containers during microwave operations. Int J Environ Res Public Health. 2013; 11(1):507-26. [DOI:10.3390/ijerph110100507] [PMID]
- Jeddi MZ, Rastkari N, Ahmadkhaniha R, Yunesian M. Concentrations of phthalates in bottled water under common storage conditions: Do they pose a health risk to children? Food Res Int. 2015; 69:256-65. [DOI:10.1016/j.foodres.2014.11.057]
- Seyhan G, Ustundag UV, Unal I, Kalkan PSA, Cansız D, Alturfan EE, et al. The effect of different storage conditions on the migration of chemicals from polyethylene terephthalate and polycarbonate bottles to water. Experimed. 2022; 12(2):74-9. [DOI:10.26650/experimed.1104796]
- Sreedhashyam H, Mehtab V, Chenna S, Upadhyayula VVR. Simultaneous determination of phthalates and bisphenols from plastic bottled water samples by dispersive solid‐phase extraction with multiwalled carbon nanotubes and liquid chromatography/atmospheric pressure photoionization/high‐resolution mass spectrometry. Rapid Commun Mass Spectrom. 2022; 36(23):e9394. [DOI:10.1002/rcm.9394] [PMID]
- Wang M, Liu Y, Liang G, Ding H, Zhou X, Qin S, et al. Migration analysis and health impact assessment of phthalates in takeaway food packaging materials. J Food Saf. 2023; 43(1):e13021. [DOI:10.1111/jfs.13021]
- Evarist EM, Chaula DN, Chove BE. Levels of phthalate acid esters in drinking water bottled in PET (Polyethylene Terephthalate) and PC (Polycarbonates) bottles-stored under different storage conditions in Mwanza City, Tanzania. Chem Sci Int Jo. 2024; 33(2):11-24. [DOI:10.9734/CSJI/2024/v33i2886]
- Zare Jeddi M, Rastkari N, Ahmadkhaniha R, Yunesian M, Nabizadeh R, Daryabeygi R. A margin of exposure approach to assessment of non-cancerous risk of diethyl phthalate based on human exposure from bottled water consumption. Environ Sci Pollut Res Int. 2015; 22(24):19518-28. [DOI:10.1007/s11356-015-5076-4] [PMID]
- Yousefi Z, Ala A, Babanezhad E, Ali Mohammadpour R. Evaluation of exposure to phthalate esters through the use of various brands of drinking water bottled in polyethylene terephthalate (PET) containers under different storage conditions. Environ. Health Eng Manag. 2019; 6(4):247-55. [DOI:10.15171/EHEM.2019.28]
- Farhoodi M, Emam-Djomeh Z, Ehsani MR, Oromiehie A. Effect of environmental conditions on the migration of di (2-ethylhexyl) phthalate from PET bottles into yogurt drinks: Influence of time, temperature, and food simulant. Arabian J Sci Eng. 2008; 33(2):279-87. [Link]
- Zaki G, Shoeib T. Concentrations of several phthalates contaminants in Egyptian bottled water: Effects of storage conditions and estimate of human exposure. Sci Total Environ. 2018; 618:142-50. [DOI:10.1016/j.scitotenv.2017.10.337] [PMID]
- Rastkari N, Jeddi MZ, Yunesian M, Ahmadkhaniha R. Effect of sunlight exposure on phthalates migration from plastic containers to packaged juices. J Environ Health Sci Eng. 2018; 16(1):27-33. [DOI:10.1007/s40201-018-0292-8] [PMID]
- Li C, Xu J, Chen D, Xiao Y. Detection of phthalates migration from disposable tablewares to drinking water using hexafluoroisopropanol-induced catanionic surfactant coacervate extraction. J Pharm Anal. 2016; 6(5):292-9. [DOI:10.1016/j.jpha.2016.04.002] [PMID]
- Fasano E, Bono-Blay F, Cirillo T, Montuori P, Lacorte S. Migration of phthalates, alkylphenols, bisphenol A and di (2-ethylhexyl) adipate from food packaging. Food Control. 2012; 27(1):132-8. [DOI:10.1016/j.foodcont.2012.03.005]
- Xu Q, Yin X, Wang M, Wang H, Zhang N, Shen Y, et al. Analysis of phthalate migration from plastic containers to packaged cooking oil and mineral water. J Agric Food Chem. 2010; 58(21):11311-7. [DOI:10.1021/jf102821h] [PMID]
- Chapa-Martínez CA, Hinojosa-Reyes L, Hernández-Ramírez A, Ruiz-Ruiz E, Maya-Treviño L, Guzmán-Mar JL. An evaluation of the migration of antimony from polyethylene terephthalate (PET) plastic used for bottled drinking water. Sci Total Environ. 2016; 565:511-8. [DOI:10.1016/j.scitotenv.2016.04.184] [PMID]
- Kazemi A, Younesi H, Bahramifar N. [Migration of bisphenol A and nonylphenol from mineral water bottles and disposable plastic containers into water at different temperatures (Persian)]. Iran J Health Environ. 2014; 6(4): 515-22. [Link]
- Nam SH, Seo YM, Kim MG. Bisphenol A migration from polycarbonate baby bottle with repeated use. Chemosphere. 2010; 79(9):949-52. [DOI:10.1016/j.chemosphere.2010.02.049] [PMID]
Article Type: Short Communication |
Subject:
Environmental Health
Received: 2024/09/27 | Accepted: 2024/11/13 | Published: 2025/01/29
Received: 2024/09/27 | Accepted: 2024/11/13 | Published: 2025/01/29
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |








