Volume 10, Issue 1 (1-2025)
CJHR 2025, 10(1): 21-36 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Akbari A, Jaafari J. The Effects of Microplastic Pollution on Marine Organisms and its Impact on Human Health: A Review. CJHR 2025; 10 (1) :21-36
URL: http://cjhr.gums.ac.ir/article-1-395-en.html
URL: http://cjhr.gums.ac.ir/article-1-395-en.html
1- Department of Environmental Health Engineering, Research Center of Health and Environment, Faculty of Health, Guilan University of Medical Sciences, Rasht, Iran.
2- Department of Environmental Health Engineering, Research Center of Health and Environment, Faculty of Health, Guilan University of Medical Sciences, Rasht, Iran. ,jalil.jaafari@yahoo.com
2- Department of Environmental Health Engineering, Research Center of Health and Environment, Faculty of Health, Guilan University of Medical Sciences, Rasht, Iran. ,
Keywords: Microplastics (MPs), Marine organisms, Marine food chain, Stress response, Inflammatory response
Full-Text [PDF 1511 kb]
(1238 Downloads)
| Abstract (HTML) (1368 Views)
Full-Text: (2099 Views)
Introduction
Marine litter has become a major problem for society, affecting all economic, social, environmental and cultural sectors [1]. Over the past few years, plastic waste has played an undeniable role in causing pollution in terrestrial, aerial, and aquatic ecosystems. They are known to be a major environmental problem, especially in aquatic environments [2]. The origin of plastics in the marine environment is mainly terrestrial, these plastics enter the marine environment through wind, rivers and sewage. Fishing and marine recreational activities are other sources of plastics in marine environments [3]. Asia produces 51% of the world’s plastic. Europe, North America, and South America produce 20%, 18%, and 4% of the world’s plastic, respectively [1]. Large plastics are broken down into smaller pieces by environmental factors, including air and ultraviolet radiation, and those that reach a size of <5 mm during the aforementioned environmental processes are called microplastics (MPs) [4]. MPs can come from primary or secondary sources. MPs that are micrometer-sized from the source are called primary MPs, such as microfibers from washing clothes. Secondary MPs are produced by the breakdown of larger plastic waste by physical, chemical, and biological processes in the environment [5]. MPs are widely distributed in marine environments. Due to their constant presence, they have the potential to be ingested by a wide range of organisms. Laboratory and environmental studies have shown that a wide range of marine organisms, from small invertebrates to large vertebrates, are susceptible to ingesting MPs with lethal or sublethal effects [6]. The toxicity of MPs arises in two ways. First, the toxicity can come from the polymer used to make plastic products. For example, polystyrene (PS), which is widely used in containers, protective packaging, bottles and lids, can be carried in the bloodstream and interfere with the reproduction of marine feeders such as many fish, some sharks, bivalves, krill and sea sponges. Second, the sharp edge and small size of MPs cause damage and inflammation in living organisms. Studies have shown that the consumption of MPs causes malnutrition and changes in reproduction for some organisms [2, 7]. Despite the negative effects of MPs on marine organisms, the presence of these tiny plastic particles can provide suitable habitats for some organisms, including water striders that require a solid substrate for reproductive activity and survival [6, 8]. Predictions suggest that by 2050, aquatic ecosystems will contain far more MPs than fish [6]. Foods and beverages are a potential route of human exposure to MPs. MPs is widely found in marine organisms including fish, crustaceans and bivalves. These marine food products appear to be the largest sources of MPs in the human diet. Human exposure to MP particles results in physical and chemical toxic effects [9, 10].
Given that most studies conducted on the effects of MPs on marine organisms specifically study one marine organism [11-14], this study reviews a set of marine organisms, considering the interactions between marine organisms and the possibility of comparing the effects of exposure to MPs in these organisms. The study describes the toxic effects of MPs in marine environments and the pollutants absorbed by them on marine organisms. In addition, it compares the effects of MPs on marine organisms in different studies, including environmental and laboratory studies, taking into account the concentration of MPS, the type and shape of MPs, and the time of exposure and finally, it assesses the risks of human exposure to MPs through edible marine organisms.
Pollution of Aquatic Environments With MPs
The increasing use of plastic materials has led to an increase in the abundance of MPs in aquatic environments. MPs can be found on sandy beaches, surface waters, in the water column, and in deep-sea sediments. Studies have also shown their presence in dead biological material (tissues and shells) and soil particles that have been blown into aquatic environments by the wind [15, 16]. The main factors influencing the abundance of MPs in the environment include population density and proximity to urban centers.
Global estimates of the occurrence of plastic waste indicate that rivers are major sources of plastic and MP pollution, transporting an average of more than 2 million tons of MPs per year [17]. Studies have also shown that riverine transport of plastic waste accounts for 80% of releases from land to marine environments [18]. MPs have become a major threat to the world’s aquatic ecosystems. Even if the introduction of new plastic waste into aquatic ecosystems is prevented, the degradation of plastics already present in the world’s aquatic ecosystems will lead to the production of MP particles on a large scale [19]. Mass production of plastic has led to the release of 4.8 to 12.7 million tons of plastic waste into the ocean [20].
Plastic polymers degrade and fragment in aquatic environments as a result of environmental processes (thermal degradation, sunlight, and biofilm growth and oxidation) [21]. Degradation refers to the breakdown of the structure of plastic polymers as a result of chemical reactions, including biodegradation, photodegradation, thermal degradation, and thermo-oxidative degradation. In the degradation process, the fragmentation of large plastic waste ultimately results in the formation of secondary MPs that are released into the environment, including aquatic environments [22].
The common chemical composition of MPs in aquatic ecosystems is mainly polypropylene (PP), polyethylene (PE), PS, polyethylene terephthalate (PET) and polyvinyl chloride (PVC) [23]. The chemical composition of MPs affects their environmental behavior. Those composed mainly of PET and PVC are more likely to settle, while PP, PE and PS float more easily. The chemical composition of MPs affects their environmental behavior. Those composed mainly of PET and PVC are more likely to settle, while PP, PE and PS float more easily [24].
The increasing presence of MP particles in the marine food chain has raised global concerns. MPs were first discovered in the guts of seabirds in the 1960s and have since been found in increasing concentrations [25]. Below, we review some of the most important marine organisms that are affected by MPs.
Effects of MPs on Marine Organisms
Plankton
Although the number of studies on MPs has increased significantly in the past few years, the number of studies investigating the effects of MPs on plankton is very low. Plankton are small organisms that are increasingly affected by MP particles because in zooplankton and ichthyoplankton the size of MPs is similar to the size of their prey, and in phytoplankton it matches their ability to entrain the particles. Since zooplankton are filter-feeder, their exposure to MP particles is high, and considering that the organism is at the beginning of the marine food chain, their exposure to MP particles causes the transmission of contamination to higher-order organisms [26, 27]. Ingestion of MPs by zooplankton depends on the particle size and concentration of MPs [28].
To investigate the effects of MPs on plankton, a number of laboratory-scale studies have been conducted in the last decade. Rodrigues et al. (2021) showed that most laboratory studies do not take into account the level of contamination present in real-world environmental conditions. In toxicology studies, it is a common problem that the researcher investigates the dose-effect relationship without considering the conditions present in the real environment. For example, MPs in marine environments that are ingested by zooplankton and ichthyoplankton are very diverse in terms of shape and polymer. While laboratory-scale studies have mostly used PS and PE microbeads, other shapes and polymers have received less attention [27].
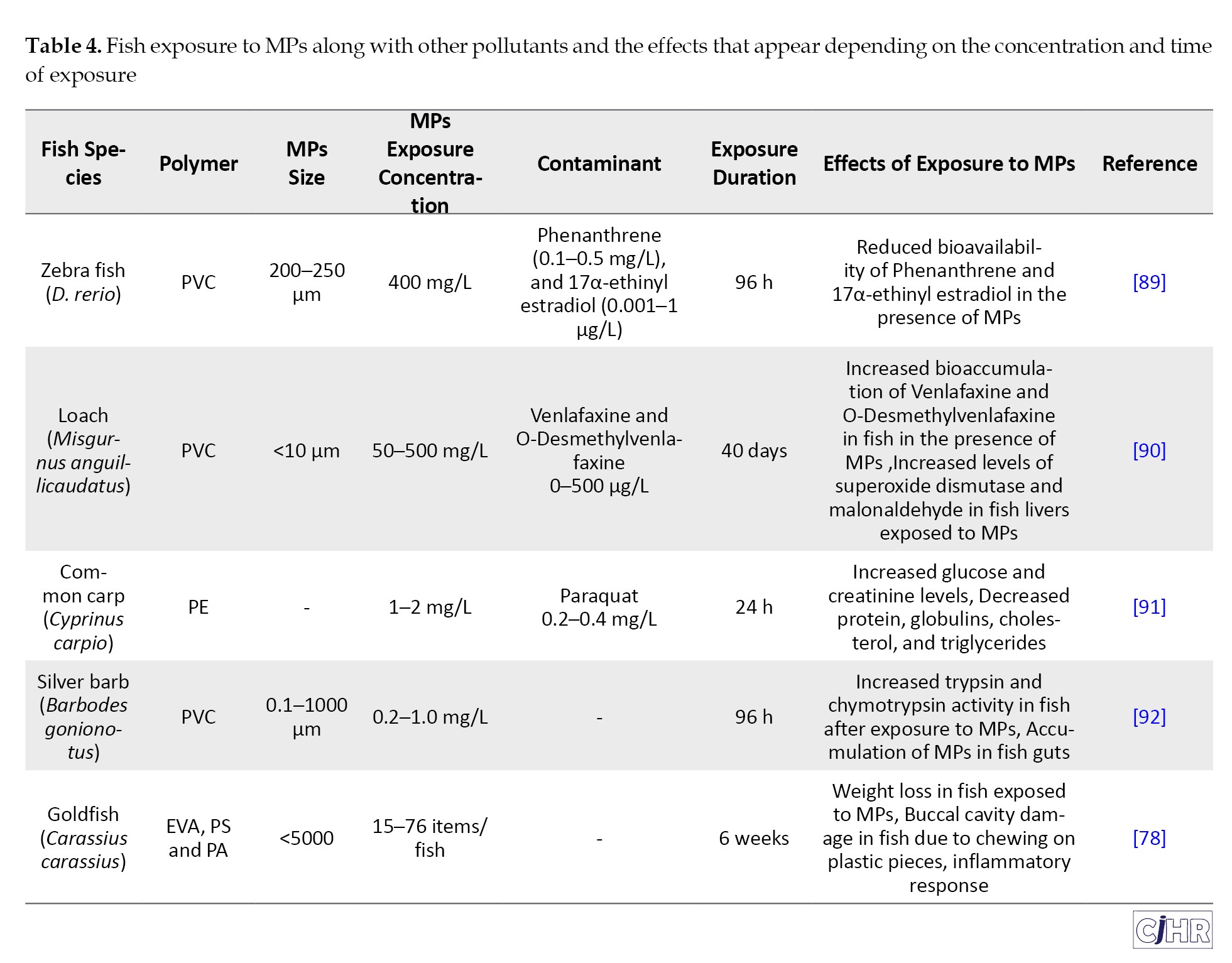
Murray and Cowie (2011) showed that the stomachs of 83% of larvae of Nephrops norvegicus, (collected from the farm), were contaminated with MP particles, with the filamentous form having the highest abundance [29]. While in the study by Zhang et al. (2019) in the East China Sea, most of the crustaceans studied were not contaminated with MPs [30]. Setala et al. (2014) showed that different species of zooplankton in the Baltic Sea (ciliates, cladocerans, polychaete larvae, copepods, worms and shrimps) were contaminated with MP particles [28]. MPs can cause reduced fecundity and changes in filtration capacity [31], feeding habits [32], swimming activity [33], and oxidative defense [34] in zooplankton. A study by Cole et al. (2013) showed that MP particles ingested by zooplankton remained in their gut for up to 7 days [35], but in a study by Watts et al. (2014) on the shore crab Carcinus maenus, the retention time of MP particles was up to 14 days [36]. Figure 1 shows the frequency of studies conducted on the effects of MPs on different plankton groups. In a study by Davarpanah et al. (2015) to investigate the effects of MPs on phytoplankton, no significant effect on growth rate was observed at the concentrations studied (0.046 to 1.472 mg/L) [37]. Considering the type and concentration of MPs, effects such as decreased lipid content, decreased cellular esterase activity, and structural changes also appear in phytoplankton [27]. Long et al. (2017) exposed Chaetoceros neogracile to PS microbeads at a concentration of (104 MPs/mL). Their results indicated that PS microbeads had no effect on the fluorescence, growth, and morphology of this phytoplankton, but when C. neogracile was exposed to a concentration of 105 and 107 MPs/mL of the same type of MP, negative effects on photosynthesis, growth, and morphology appeared [38]. Table 1 shows the abundance and characteristics of MPs in plankton samples from different studies.
Bivalve
MPs can have detrimental effects on the feeding and reproductive behavior and filtration activity of bivalves. MPs can also have detrimental effects on bivalves by damaging their food sources, altering the structure of their sedimentary habitats, and releasing persistent organic pollutants [47]. In order to understand the effects of MPs on bivalves, especially species that are consumed by humans, it is necessary to investigate the routes of exposure of these organisms to MPs particles.
It is likely that the abundance of MP particles in marine environments and the lack of sufficient food are the causes of the ingestion of MPs by these organisms. It is also likely that mussels ingest fewer MPs than other marine invertebrates. Processing of food particles in bivalves occurs after ingestion. Preingestive sorting results in the formation of pseudofeces, (material rejected prior to ingestion). The sorting process before ingestion increases the quality of ingested nutrients [48].
The first damage caused by MPs to bivalves is clogging of the gills and digestive tract. MP particles have the ability to accumulate in the digestive tract, valves, gills, and hepatopancreas of this organism. MPs were found in the forms of fibers, films, and fragments of bivalves, with fibers being the most abundant among these forms [49, 50]. More than 80 percent of MP particles found in mussels collected from coastal waters in China were in the form of fibers [51]. The harmful effects of MPs on bivalves are greater in the form of fibers than in other forms, because they are more difficult to remove from the digestive tract of this organism, which causes it to be in contact with MP particles and pollutants attached to them for a longer period of time [52].
Since the unit of quantification of MP particles varies in different studies, it is difficult to compare them with each other. The abundance of MPs was expressed as 57.17±17.34 particles per individual in Patinopecten yessoensis [52]. But in another study conducted on Mytilus galloprovincialis, this frequency was 6.2 to 7.2 particles per gram of the organism’s body [50]. Smaller MPs have a higher residence time and greater potential for accumulation in bivalve tissues [47].
Wegner et al. (2012) showed that exposure to 30 nm PS beads (0.1–0.3 g/L) negatively affected filtration activity in the blue mussel Mytilus edulis [53]. Rist et al. (2016) similarly showed that exposure to 1–50 μm PVC (216 mg/L) MPs also resulted in reduced filtration activity in the Asian green mussel Perna viridis [54].
Guillermino et al. (2018) showed that MPs have the ability to inhibit the enzyme acetylcholinesterase in Corbicula fluminea, causing neurotoxicity [55]. In the study by Avio et al., (2015), exposure to 50 μg/L of PE and PS MPs for 7 days inhibited the enzyme acetylcholinesterase in M. galloprovincialis [56]. Acetylcholinesterase is an enzyme that hydrolyzes acetylcholine at the synapse. By degrading acetylcholine, acetylcholinesterase prevents this contraction from being prolonged. MPs can also disrupt the reproduction of bivalves. In the study by Sussarellu et al. (2016), exposure to PS MPs at 23 μg/L reduced oocyte diameter and number in female Crassostrea gigas oysters. Reduced oocyte number and diameter decrease the probability of larval survival in oysters [57].
Marine predators, as well as humans, use bivalves as a food source, and it is possible that these MPs can be transferred from contaminated bivalves to humans and the predators that feed on them. Studies have confirmed the presence of MP particles in the tissues of bivalves farmed for human consumption in the Atlantic Ocean and the North Sea [58]. Table 2 shows the exposure of bivalves to MPs and the effects that appear, considering concentration and exposure time.

Corals
Coral reefs are an essential and irreplaceable ecological treasure. Coral reefs, among the richest marine habitats, provide the livelihoods of more than 275 million people. They are constantly threatened by global warming, ocean acidification, overfishing, and coastal development [64].
Corals have the highest biodiversity in the marine environment. Coral reefs protect the coast from destructive ocean currents [65]. Although MP pollution and its destructive effects on coral reefs have attracted considerable attention, not much information is available about the occurrence, fate, and impact of MPs on coral reefs. Researchers have gradually begun to investigate the abundance and characteristics of MPs in coral reef areas over the past few years [66, 67].
Corals obtain their energy through photosynthesis from symbiotic algae in their tissues, and they also have the ability to consume small marine organisms such as phytoplankton and zooplankton. In marine environments, MPs can be ingested by marine organisms such as corals, which can affect coral health. This is because MPs are not digested by corals and remain in their digestive systems [68]. Hall et al. (2015) showed that blue PP MPs (10–2000 μm) were consumed at a rate of 1.2–55 μg/cm2 h by the stony coral Dipsastrea pallida and persisted in the mesenterial tissue of the coral gut cavity for more than 24 h [69]. There are still many uncertainties regarding the mechanism of MP ingestion by corals. Using current knowledge, it can be said that the weathering process of MPs and the presence of natural food can affect the ingestion of MPs by corals [13].
Corona et al. (2020) showed that microbial biofilm attached to PE MPs increases the likelihood of ingestion and accumulation of these MPs by the mushroom coral Danafungia scruposa, as corals mistakenly consume MPs saturated with microbial biofilms as natural food [70]. Studies have shown that coral exposure to MPs can have negative effects on coral growth and health. MPs can lead to reduced skeletal calcification, tissue necrosis, reduced photosynthesis, impaired feeding behavior, and impaired energy expenditure [13].
Mendrik et al. (2021) investigated the effects of various forms of MPs on two coral species, Acropora and Seriatopora hysterix. They showed that fibrous MPs affected coral feeding behavior and induced stress responses. Exposing Acropora to MP fibers for 12 days reduced photosynthetic activity in this organism by about 41%. [71].
Hankins et al. (2021) investigated the effects of MP toxicity on two stony coral species, Pseudodiploria clivosa and Acropora cervicornis, which are common species in the Atlantic Ocean. Prolonged exposure to PE MPs reduced calcification, thereby reducing coral skeletal growth [72]. Among the different types of MPs, PVC MPs induced greater toxicity than other polymers, and smaller MPs also had higher retention times and greater disruption than larger sized MPs [19].
In the study conducted by Cordova (2018) on the investigation of MPs in coral sediments in Sekotong, Lombok Island of Indonesia, the average concentration of MPs was 48.3±13.98 items/kg, the most abundant type was PS, and the most common shapes of MPs were foam, fragments, and granules [73]. Studies have shown that Astrangia poculata corals prefer to ingest PE beads (170.5–230.8 μm), which reduces the organism’s natural food intake, brine shrimp eggs, due to a false sense of satiety, with potential impacts on coral health [13]. Table 3 shows the exposure of corals to MPs and the effects that appear, considering concentration and exposure time.

Fish
Ingestion of MPs by fish in marine environments can occur through contaminated bait, the water column, and also through bottom sediments [76]. The presence of MP particles mixed with sediment, as well as the presence of MPs suspended in the water column, leads to unintentional ingestion of MP particles by fish when they are foraging. In general, in fish, the amount of MP ingested increases with increasing fish size. This relationship exists until a certain maximum for the size of the plastic and the fish is reached, after which this relationship between the size of the fish and the amount of plastic ingested may no longer exist [77]. The size and amount of MPs that fish ingest are influenced by their feeding behavior. Omnivorous fish feed on both animal and plant food sources and consume a wider range of food, and therefore have a higher exposure to MP particles compared to herbivorous and carnivorous fish [76].
Results from analysis of the digestive tracts of various fish species confirm the presence of MP particles in the digestive tracts of these organisms. After ingestion, MPs accumulate in the digestive tract of fish, causing blockage of the digestive system and reduced feeding due to false satiety [12]. Ingestion of MPs can disrupt the structure and function of the digestive tract, leading to nutritional and growth problems in fish [78]. The color of MPs is a physical characteristic that influences the uptake of MPs in the marine food chain. Dark-colored MPs (black, blue, and green) are ingested by fish in greater amounts than light-colored MPs, likely due to their resemblance to real prey [79].
Yin et al. (2018) showed that exposure of Jacopever fish to PS MPs at a concentration of 106 particles/L reduced the weight gain rate by 65.4%, the growth rate by 65.9%, and the gross energy of fish by 9.5% [80]. Other effects of MPs in fish include inflammatory responses and immune system disorders [12]. Laboratory studies support the hypothesis that exposure to MPs results in toxicological effects in fish. The question here is whether toxicological effects also occur under environmental conditions [81]. This is because in the environment, organisms are exposed to a mixture of MPs, but most studies conducted to investigate the toxicity effects of MPs expose organisms to only one type of MP. Also, most studies used microbeads to investigate toxicity effects, while in marine environments, the predominant form of MPs is fibers. Also, concentrations of MPs in marine environments are very different from the concentrations that fish were exposed to in laboratory conditions. In the composition of plastics, there are some additives such as bisphenol A (BPA) and nonylphenol, which are used to improve the properties of the polymer. There is a possibility that the additives can leak from MPs and enter aquatic environments and fish [12].
Just as fibers and fragments are the most common forms of MPs in aquatic environments, they are also the most common forms of MPs detected in fish [82]. PP, polyester (PES), PE, and PS are the most abundant polymers found worldwide. These polymers were also the most abundant in the digestive tract of fish [83].
Alomar et al. (2017), who studied the presence of MP particles in the stomachs blackmouth catshark (Galeus melastomus) in the Mediterranean Sea, found high concentrations of MPs in the stomachs of this animal [82]. Most of these MPs remain in the fish’s stomach and intestines after ingestion. MP particles also have the ability to enter the circulatory or lymphatic system, where they can be distributed throughout the body. They also have the ability to stick to fish skin [84]. Figure 2 shows the entry of MPs into marine ecosystems due to human activities and its effects on marine organisms. Nerland et al. (2014) studied Japanese medaka (Oryzias latipes) over a long period of time, and their results showed that moderate concentrations (8 ngL−1 of PE of size <0.5 mm) of MPs in the sea (San Diego Bay, California) can have harmful but non-lethal effects on the fish [85]. In a study by Ramos et al. (2012) in a tropical river in northeastern Brazil, bottom-feeding fish (Gerreidae) in this river were found to be contaminated with MPs and their stomachs were severely affected by MP particles [86].
Batel et al. (2016) artificially simulated a food chain in an aquatic environment using brine shrimp (Artemia sp.) and zebrafish (Danio rerio) and concluded that MPs have the ability to transfer benzo(a)pyrene from brine shrimp (Artemia sp.) to zebrafish (D. rerio). This study demonstrated that fish may be exposed to contaminants attached to MP particles through ingestion of other marine organisms contaminated with MPs [87]. MPs may also carry pathogens and antibiotic resistance genes that may cause disease after being ingested by fish [88]. Table 4 shows the exposure of fish to MPs along with other pollutants and the effects that appear depending on the concentration and time of exposure.
Sea birds
In 2023, plasticosis was discovered in seabirds, a new disease caused solely by plastic. Birds identified as suffering from this disease had their digestive tracts injured by ingestion of plastic debris [93]. Following ingestion of small pieces of plastic, their digestive tracts become inflamed. Over time, persistent inflammation causes tissues to scar and deform, affecting digestion, growth, and survival [94].
Seabirds, including shearwaters, northern fulmars, petrels, albatrosses feed on the surface of the sea and ingest MP particles floating in the water. Ingested MPs accumulate and concentrate in the stomachs of seabirds. In studies, 30-35 percent of the plastics detected in the birds’ bodies were in the form of industrial pellets [68, 95, 96]. In the South Atlantic region, plastic pellets were also found in the digestive tracts of seabirds, which excreted the plastic along with their feces [97]. In a study by Vlietstra et al. (2002) on the short-tailed shearwater, which lives in the North Sea, MP particles were also found in the digestive tract of this bird [98].
Carlin et al. (2020) found MP particles in the digestive tracts of all species of birds of prey in Central Florida. The average total MPs in birds and per gram of bird digestive tract tissue were 11.9 and 0.3, respectively. In this study, the fiber form accounted for more than 86 percent of all plastics. Most of the fibers were clear or blue in color [99]. Seabirds, including Larus glaucescens, can remove MP particles from their digestive tracts through a process called regurgitation [100]. Studies have shown that younger northern fulmars (Fulmarus glacialis) ingest more MPs during the feeding process, which accumulate in their intestines. Ingesting MP particles causes hunger and loss of fitness [101].
Bilal et al. (2023) identified a total of 2033 MP particles in 20 samples of ducks (Anas platyrhynchos) from the Panjkora River. The mean abundance of MPs was 44.6±15.8 MPs in the crop and 57.05±18.7 MPs in the gizzard. Ducks are constantly exposed to MP pollution due to their proximity to polluted aquatic ecosystems and their feeding habits [102]. Figure 3 shows MPs contamination in seabirds and the transfer of MPs in the food chain.
Marine mammal and turtles
The effects of MPs have been studied in many large marine organisms, including harbor seals, sea turtles, polar bears, and whales [103]. Marine mammals can ingest MPs directly in the marine environment or indirectly through ingestion of smaller marine organisms that have consumed the MPs [6]. Ingested MPs may be excreted through pseudofaece or accumulate in the organism and move between tissues [4, 56].
The likelihood of ingesting MP particles and their accumulation in the stomach and intestines of whales is high, due to the high fat and lipid content of this animal. There are many reports of the death of stranded whales with intestines full of MP particles. Although there have been no reports of polar bears ingesting MPs, the likelihood of this creature ingesting MP particles is high [68]. In a study by Nerland et al. (2014), 60.5% of sea turtles showed accumulation of MP particles in their digestive tract [85].
Studies have shown that MP particles in gray seals living in a sanctuary where human activity and pollution were minimal, indicating the presence of MPs even in controlled environmental conditions [104]. As MP particles get smaller, they are more likely to be ingested by larger marine creatures. Whales (Megaptera novaeangliae) filter small food particles from large volumes of water, which means they ingest large amounts of MPs along with their food [105].
Examination of the gut contents of baleen whales shows that their diet often consisted of mesopelagic fish, cephalopods, and MPs, and MP particles were found throughout the whale’s digestive tract. It is likely that MPs in the whale’s digestive tract are eventually excreted. The stomachs of some baleen whales, including True’s beaked whale Mesoplodon mirus and Cuvier’s beaked whale Ziphius cavirostris, contained only plastic particles and no food was found in their stomachs [106]. The question now is whether whales are able to excrete ingested plastic particles and whether these plastic particles can cause blockages in their digestive tracts. It has yet to be determined whether some whales, such as filter feeding baleen whales, are able to excrete ingested plastic particles while some others, including beaked whales, are unable to excrete the plastic fragments they ingest. Additionally, there is considerable uncertainty about whether the size of ingested plastic can affect a whale’s ability to excrete plastic and whether size plays a role in gastrointestinal blockage [76]. Getting trapped in plastic waste and ingesting MPs causes acute and chronic damage and increases the pollutant load in the cetaceans, ultimately leading to their death [6].
Fiber is the most abundant form of MPs in the digestive tract of marine mammals [107]. MPs in various colors, such as black, white, green, and blue, have been reported in the digestive tract and feces of marine mammals [107, 108]. Among the polymers identified in marine mammals, nylon, cotton, PP, polyethersulfone, ethylene propylene, and polyester were the most abundant [109].
MPs have become a serious threat to marine mammals [110]. Consumption of MPs can reduce energy reserves, feeding capacity and reproduction. MPs can cause adverse changes in the physiology of the gastrointestinal tract and suppress the immune system. Vulnerability to diseases, oxidative stress and cytotoxicity are other effects of exposure to MPs [104, 111].
The nature of MPs determines the extent of bioaccumulation of pollutants in different tissues. MPs are likely to cross cell membranes and the blood-brain barrier in marine mammals, they can enter the bloodstream and concentrate in other vital internal organs such as the placenta, liver, and kidney [112].
MPs in marine environments provide a highly selective and flexible support platform for a variety of microbial communities, especially resistant pathogens [113]. Pathogens accumulated on MPs can travel long distances by waves, currents, and winds and be transmitted to different hosts, thereby causing disease outbreaks [114]. The concentration of antimicrobial-resistant pathogens on the surface of MPs is 100 to 5000 times higher than in the surrounding water [88]. Marine mammals are predators and may consume high concentrations of MPs through the food web, but to date, no studies have been conducted on whether pathogen-laden MPs can transmit pathogens and cause disease in marine mammals. Microorganisms attached to MPs use the adsorbed metals (e.g. cobalt, iron, zinc, manganese, copper) as micronutrients. Many pathogens require these metals for survival and pathogenicity [115]. MPs can disrupt the protective lung barriers of marine mammals, allowing opportunistic pathogens to penetrate deep into the lungs, leading to respiratory infections [114]. It was proven that marine mammals whose cause of death was infectious disease had much higher MPs among the factors leading to mortality [116].
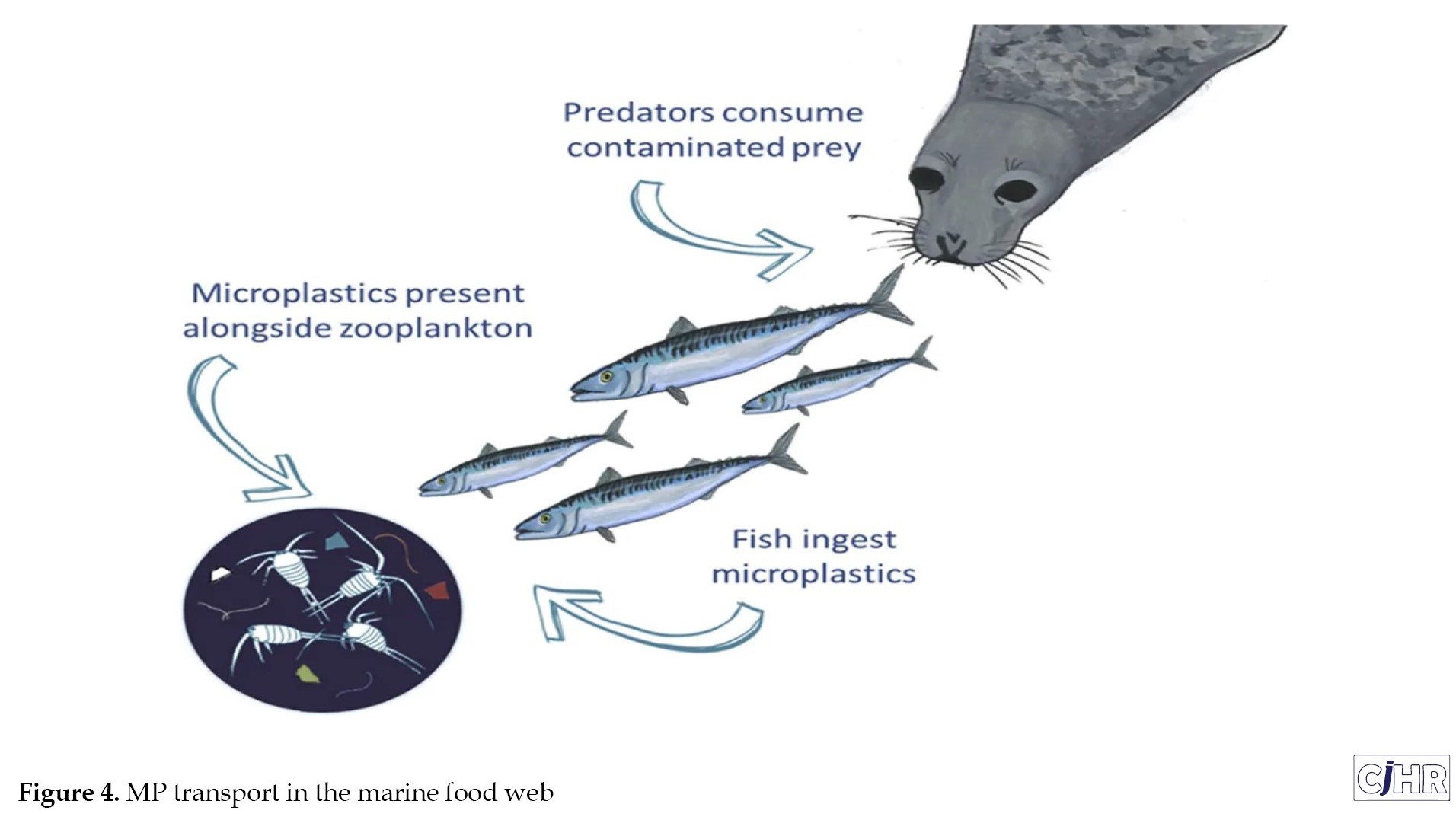
Studies show that atmospheric deposition transports MP particles into the ocean surface air, so marine mammals and sea turtles that breathe ocean surface air are susceptible to inhaling MP particles. Inhaling airborne MPs can cause significant harm to sea turtles and marine mammals, as they inhale large amounts of air when breathing and hold their breath during prolonged diving in the seas and oceans, which increases the duration of exposure to MPs and other pollutants in the air [109]. Figure 4 shows the transport of MPs in the marine food web.
Human exposure to MPs through seafood diet
The accumulation of MPs in marine organisms consumed as human food leads to dangerous consequences for human health. Commercial marine organisms such as crab, oyster, fish, and sea cucumber ingest MP particles and transport them throughout the food web [68].
To identify the abundance of MPs in marine environments and marine organisms, appropriate indicator species are needed to assess MP pollution, exposure to MPs, and environmental and human risks [117]. Mussels (Mytilus spp.) are one of the marine indicator organisms for the assessment of the above. They are able to tolerate different environmental conditions and are widely distributed in marine environments. Mussels are also filter feeders and are directly exposed to MP particles, so the concentration of MPs in mussel tissues has a close relationship with the concentration of MPs in marine environments. Mollusks are the most important route of human exposure to MPs through the marine diet, as these organisms are fully consumed by humans, making them a good indicator of MP contamination and the safety of seafood consumed by humans [118, 119].
Research by Lia et al. (2018) shows that mussels collected from different areas, including coastal waters and supermarkets, are contaminated with MP particles and predicted that consumers eat 70 MPs in 100 grams of mussels [120]. MPs were also detected in sardines and sprats cans [121] and the organs (e.g. stomach contents and liver) of fish Squalius cephalus [122]. It has also been reported that consuming Mud carp (Cirrhinus molitorella) and Bighead carp (Hypophthalmichthys nobilis) have the potential risk of accumulating phthalate esters along gastrointestinal tract [123].
Young fish, arthropods, and mollusks that are prey to shrimp may be contaminated with MP particles. These MP particles accumulate in the digestive tract of shrimp after ingestion of the aforementioned organisms. Given that shrimp are consumed whole by humans without removing the digestive tract, consumption of shrimp is a potential route for human exposure to MPs through seafood [119].
Human exposure to MP particles results in physical and chemical toxic effects. The sensitivity of the exposed individual and the type of MP particles are factors influencing the development of adverse effects. The physical effects may have different concerning impacts, including enhanced toxicity, cell damage, inflammatory response, and oxidative stress [10]. Since the immune system cannot eliminate MPs particles, these particles lead to chronic inflammation and an increased risk of neoplasia [124]. The chemical effects of MP particles on humans are caused either by the absorption of pollutants from the environment (PAH, PCB, and toxic metals) into MPs or by the chemicals used in the production of plastics [60]. Among these chemicals, BPA and phthalates cause endocrine disruption, neurotoxic effects, and cancer in humans [125]. Most of the information on MPs in the marine food web is related to their presence in the digestive tract of seafood, while in many cases the digestive tract of marine organisms is discarded before consumption by humans [126]. Also, when assessing the risk of MPs to human health, it is necessary to consider the risk of pathogenic microorganisms adsorbed to MP particles, as there is a possibility of enhanced growth and colony formation of pathogens in the MP substrate [119].
Conclusion
Over the past few years, plastic waste has played an undeniable role in causing pollution in terrestrial, aerial, and aquatic ecosystems. They are known to be a major environmental problem, especially in aquatic environments. Plastic polymers degrade and fragment in aquatic environments as a result of environmental processes (thermal degradation, sunlight, and biofilm growth and oxidation), and those that reach a size of <5 mm are called MPs. The presence of MPs in marine environments and their transfer through the marine food chain has endangered a wide range of marine organisms, including plankton, bivalves, corals, fish, seabirds, and marine mammals. Exposure of marine organisms to MPs can lead to weight loss, reduced growth, reduced reproduction, changes in feeding behavior, changes in enzyme activity, initiation of stress response, and inflammatory response. Atmospheric deposition transports MP particles into the ocean surface air, so marine mammals and sea turtles that breathe ocean surface air are susceptible to inhaling MP particles in addition to ingesting them. The accumulation of MPs in marine organisms consumed as human food leads to dangerous consequences for human health. Commercial marine organisms such as crab, oyster, fish, and sea cucumber ingest MP particles and transport them throughout the food web. Human exposure to MP particles results in physical and chemical toxic effects.
Ethical Considerations
Compliance with ethical guidelines
There were no ethical considerations to be considered in this work.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
All authors equally contributed to preparing this article.
Conflict of interest
The authors declared no conflict of interest
Acknowledgements
The authors are most grateful to the Department of Environmental Health Engineering, School of Public Health, Guilan University of Medical Sciences, Rasht, Iran, for their collaboration in this research.
References
Marine litter has become a major problem for society, affecting all economic, social, environmental and cultural sectors [1]. Over the past few years, plastic waste has played an undeniable role in causing pollution in terrestrial, aerial, and aquatic ecosystems. They are known to be a major environmental problem, especially in aquatic environments [2]. The origin of plastics in the marine environment is mainly terrestrial, these plastics enter the marine environment through wind, rivers and sewage. Fishing and marine recreational activities are other sources of plastics in marine environments [3]. Asia produces 51% of the world’s plastic. Europe, North America, and South America produce 20%, 18%, and 4% of the world’s plastic, respectively [1]. Large plastics are broken down into smaller pieces by environmental factors, including air and ultraviolet radiation, and those that reach a size of <5 mm during the aforementioned environmental processes are called microplastics (MPs) [4]. MPs can come from primary or secondary sources. MPs that are micrometer-sized from the source are called primary MPs, such as microfibers from washing clothes. Secondary MPs are produced by the breakdown of larger plastic waste by physical, chemical, and biological processes in the environment [5]. MPs are widely distributed in marine environments. Due to their constant presence, they have the potential to be ingested by a wide range of organisms. Laboratory and environmental studies have shown that a wide range of marine organisms, from small invertebrates to large vertebrates, are susceptible to ingesting MPs with lethal or sublethal effects [6]. The toxicity of MPs arises in two ways. First, the toxicity can come from the polymer used to make plastic products. For example, polystyrene (PS), which is widely used in containers, protective packaging, bottles and lids, can be carried in the bloodstream and interfere with the reproduction of marine feeders such as many fish, some sharks, bivalves, krill and sea sponges. Second, the sharp edge and small size of MPs cause damage and inflammation in living organisms. Studies have shown that the consumption of MPs causes malnutrition and changes in reproduction for some organisms [2, 7]. Despite the negative effects of MPs on marine organisms, the presence of these tiny plastic particles can provide suitable habitats for some organisms, including water striders that require a solid substrate for reproductive activity and survival [6, 8]. Predictions suggest that by 2050, aquatic ecosystems will contain far more MPs than fish [6]. Foods and beverages are a potential route of human exposure to MPs. MPs is widely found in marine organisms including fish, crustaceans and bivalves. These marine food products appear to be the largest sources of MPs in the human diet. Human exposure to MP particles results in physical and chemical toxic effects [9, 10].
Given that most studies conducted on the effects of MPs on marine organisms specifically study one marine organism [11-14], this study reviews a set of marine organisms, considering the interactions between marine organisms and the possibility of comparing the effects of exposure to MPs in these organisms. The study describes the toxic effects of MPs in marine environments and the pollutants absorbed by them on marine organisms. In addition, it compares the effects of MPs on marine organisms in different studies, including environmental and laboratory studies, taking into account the concentration of MPS, the type and shape of MPs, and the time of exposure and finally, it assesses the risks of human exposure to MPs through edible marine organisms.
Pollution of Aquatic Environments With MPs
The increasing use of plastic materials has led to an increase in the abundance of MPs in aquatic environments. MPs can be found on sandy beaches, surface waters, in the water column, and in deep-sea sediments. Studies have also shown their presence in dead biological material (tissues and shells) and soil particles that have been blown into aquatic environments by the wind [15, 16]. The main factors influencing the abundance of MPs in the environment include population density and proximity to urban centers.
Global estimates of the occurrence of plastic waste indicate that rivers are major sources of plastic and MP pollution, transporting an average of more than 2 million tons of MPs per year [17]. Studies have also shown that riverine transport of plastic waste accounts for 80% of releases from land to marine environments [18]. MPs have become a major threat to the world’s aquatic ecosystems. Even if the introduction of new plastic waste into aquatic ecosystems is prevented, the degradation of plastics already present in the world’s aquatic ecosystems will lead to the production of MP particles on a large scale [19]. Mass production of plastic has led to the release of 4.8 to 12.7 million tons of plastic waste into the ocean [20].
Plastic polymers degrade and fragment in aquatic environments as a result of environmental processes (thermal degradation, sunlight, and biofilm growth and oxidation) [21]. Degradation refers to the breakdown of the structure of plastic polymers as a result of chemical reactions, including biodegradation, photodegradation, thermal degradation, and thermo-oxidative degradation. In the degradation process, the fragmentation of large plastic waste ultimately results in the formation of secondary MPs that are released into the environment, including aquatic environments [22].
The common chemical composition of MPs in aquatic ecosystems is mainly polypropylene (PP), polyethylene (PE), PS, polyethylene terephthalate (PET) and polyvinyl chloride (PVC) [23]. The chemical composition of MPs affects their environmental behavior. Those composed mainly of PET and PVC are more likely to settle, while PP, PE and PS float more easily. The chemical composition of MPs affects their environmental behavior. Those composed mainly of PET and PVC are more likely to settle, while PP, PE and PS float more easily [24].
The increasing presence of MP particles in the marine food chain has raised global concerns. MPs were first discovered in the guts of seabirds in the 1960s and have since been found in increasing concentrations [25]. Below, we review some of the most important marine organisms that are affected by MPs.
Effects of MPs on Marine Organisms
Plankton
Although the number of studies on MPs has increased significantly in the past few years, the number of studies investigating the effects of MPs on plankton is very low. Plankton are small organisms that are increasingly affected by MP particles because in zooplankton and ichthyoplankton the size of MPs is similar to the size of their prey, and in phytoplankton it matches their ability to entrain the particles. Since zooplankton are filter-feeder, their exposure to MP particles is high, and considering that the organism is at the beginning of the marine food chain, their exposure to MP particles causes the transmission of contamination to higher-order organisms [26, 27]. Ingestion of MPs by zooplankton depends on the particle size and concentration of MPs [28].
To investigate the effects of MPs on plankton, a number of laboratory-scale studies have been conducted in the last decade. Rodrigues et al. (2021) showed that most laboratory studies do not take into account the level of contamination present in real-world environmental conditions. In toxicology studies, it is a common problem that the researcher investigates the dose-effect relationship without considering the conditions present in the real environment. For example, MPs in marine environments that are ingested by zooplankton and ichthyoplankton are very diverse in terms of shape and polymer. While laboratory-scale studies have mostly used PS and PE microbeads, other shapes and polymers have received less attention [27].
Murray and Cowie (2011) showed that the stomachs of 83% of larvae of Nephrops norvegicus, (collected from the farm), were contaminated with MP particles, with the filamentous form having the highest abundance [29]. While in the study by Zhang et al. (2019) in the East China Sea, most of the crustaceans studied were not contaminated with MPs [30]. Setala et al. (2014) showed that different species of zooplankton in the Baltic Sea (ciliates, cladocerans, polychaete larvae, copepods, worms and shrimps) were contaminated with MP particles [28]. MPs can cause reduced fecundity and changes in filtration capacity [31], feeding habits [32], swimming activity [33], and oxidative defense [34] in zooplankton. A study by Cole et al. (2013) showed that MP particles ingested by zooplankton remained in their gut for up to 7 days [35], but in a study by Watts et al. (2014) on the shore crab Carcinus maenus, the retention time of MP particles was up to 14 days [36]. Figure 1 shows the frequency of studies conducted on the effects of MPs on different plankton groups. In a study by Davarpanah et al. (2015) to investigate the effects of MPs on phytoplankton, no significant effect on growth rate was observed at the concentrations studied (0.046 to 1.472 mg/L) [37]. Considering the type and concentration of MPs, effects such as decreased lipid content, decreased cellular esterase activity, and structural changes also appear in phytoplankton [27]. Long et al. (2017) exposed Chaetoceros neogracile to PS microbeads at a concentration of (104 MPs/mL). Their results indicated that PS microbeads had no effect on the fluorescence, growth, and morphology of this phytoplankton, but when C. neogracile was exposed to a concentration of 105 and 107 MPs/mL of the same type of MP, negative effects on photosynthesis, growth, and morphology appeared [38]. Table 1 shows the abundance and characteristics of MPs in plankton samples from different studies.

Bivalve
MPs can have detrimental effects on the feeding and reproductive behavior and filtration activity of bivalves. MPs can also have detrimental effects on bivalves by damaging their food sources, altering the structure of their sedimentary habitats, and releasing persistent organic pollutants [47]. In order to understand the effects of MPs on bivalves, especially species that are consumed by humans, it is necessary to investigate the routes of exposure of these organisms to MPs particles.
It is likely that the abundance of MP particles in marine environments and the lack of sufficient food are the causes of the ingestion of MPs by these organisms. It is also likely that mussels ingest fewer MPs than other marine invertebrates. Processing of food particles in bivalves occurs after ingestion. Preingestive sorting results in the formation of pseudofeces, (material rejected prior to ingestion). The sorting process before ingestion increases the quality of ingested nutrients [48].
The first damage caused by MPs to bivalves is clogging of the gills and digestive tract. MP particles have the ability to accumulate in the digestive tract, valves, gills, and hepatopancreas of this organism. MPs were found in the forms of fibers, films, and fragments of bivalves, with fibers being the most abundant among these forms [49, 50]. More than 80 percent of MP particles found in mussels collected from coastal waters in China were in the form of fibers [51]. The harmful effects of MPs on bivalves are greater in the form of fibers than in other forms, because they are more difficult to remove from the digestive tract of this organism, which causes it to be in contact with MP particles and pollutants attached to them for a longer period of time [52].
Since the unit of quantification of MP particles varies in different studies, it is difficult to compare them with each other. The abundance of MPs was expressed as 57.17±17.34 particles per individual in Patinopecten yessoensis [52]. But in another study conducted on Mytilus galloprovincialis, this frequency was 6.2 to 7.2 particles per gram of the organism’s body [50]. Smaller MPs have a higher residence time and greater potential for accumulation in bivalve tissues [47].
Wegner et al. (2012) showed that exposure to 30 nm PS beads (0.1–0.3 g/L) negatively affected filtration activity in the blue mussel Mytilus edulis [53]. Rist et al. (2016) similarly showed that exposure to 1–50 μm PVC (216 mg/L) MPs also resulted in reduced filtration activity in the Asian green mussel Perna viridis [54].
Guillermino et al. (2018) showed that MPs have the ability to inhibit the enzyme acetylcholinesterase in Corbicula fluminea, causing neurotoxicity [55]. In the study by Avio et al., (2015), exposure to 50 μg/L of PE and PS MPs for 7 days inhibited the enzyme acetylcholinesterase in M. galloprovincialis [56]. Acetylcholinesterase is an enzyme that hydrolyzes acetylcholine at the synapse. By degrading acetylcholine, acetylcholinesterase prevents this contraction from being prolonged. MPs can also disrupt the reproduction of bivalves. In the study by Sussarellu et al. (2016), exposure to PS MPs at 23 μg/L reduced oocyte diameter and number in female Crassostrea gigas oysters. Reduced oocyte number and diameter decrease the probability of larval survival in oysters [57].
Marine predators, as well as humans, use bivalves as a food source, and it is possible that these MPs can be transferred from contaminated bivalves to humans and the predators that feed on them. Studies have confirmed the presence of MP particles in the tissues of bivalves farmed for human consumption in the Atlantic Ocean and the North Sea [58]. Table 2 shows the exposure of bivalves to MPs and the effects that appear, considering concentration and exposure time.

Corals
Coral reefs are an essential and irreplaceable ecological treasure. Coral reefs, among the richest marine habitats, provide the livelihoods of more than 275 million people. They are constantly threatened by global warming, ocean acidification, overfishing, and coastal development [64].
Corals have the highest biodiversity in the marine environment. Coral reefs protect the coast from destructive ocean currents [65]. Although MP pollution and its destructive effects on coral reefs have attracted considerable attention, not much information is available about the occurrence, fate, and impact of MPs on coral reefs. Researchers have gradually begun to investigate the abundance and characteristics of MPs in coral reef areas over the past few years [66, 67].
Corals obtain their energy through photosynthesis from symbiotic algae in their tissues, and they also have the ability to consume small marine organisms such as phytoplankton and zooplankton. In marine environments, MPs can be ingested by marine organisms such as corals, which can affect coral health. This is because MPs are not digested by corals and remain in their digestive systems [68]. Hall et al. (2015) showed that blue PP MPs (10–2000 μm) were consumed at a rate of 1.2–55 μg/cm2 h by the stony coral Dipsastrea pallida and persisted in the mesenterial tissue of the coral gut cavity for more than 24 h [69]. There are still many uncertainties regarding the mechanism of MP ingestion by corals. Using current knowledge, it can be said that the weathering process of MPs and the presence of natural food can affect the ingestion of MPs by corals [13].
Corona et al. (2020) showed that microbial biofilm attached to PE MPs increases the likelihood of ingestion and accumulation of these MPs by the mushroom coral Danafungia scruposa, as corals mistakenly consume MPs saturated with microbial biofilms as natural food [70]. Studies have shown that coral exposure to MPs can have negative effects on coral growth and health. MPs can lead to reduced skeletal calcification, tissue necrosis, reduced photosynthesis, impaired feeding behavior, and impaired energy expenditure [13].
Mendrik et al. (2021) investigated the effects of various forms of MPs on two coral species, Acropora and Seriatopora hysterix. They showed that fibrous MPs affected coral feeding behavior and induced stress responses. Exposing Acropora to MP fibers for 12 days reduced photosynthetic activity in this organism by about 41%. [71].
Hankins et al. (2021) investigated the effects of MP toxicity on two stony coral species, Pseudodiploria clivosa and Acropora cervicornis, which are common species in the Atlantic Ocean. Prolonged exposure to PE MPs reduced calcification, thereby reducing coral skeletal growth [72]. Among the different types of MPs, PVC MPs induced greater toxicity than other polymers, and smaller MPs also had higher retention times and greater disruption than larger sized MPs [19].
In the study conducted by Cordova (2018) on the investigation of MPs in coral sediments in Sekotong, Lombok Island of Indonesia, the average concentration of MPs was 48.3±13.98 items/kg, the most abundant type was PS, and the most common shapes of MPs were foam, fragments, and granules [73]. Studies have shown that Astrangia poculata corals prefer to ingest PE beads (170.5–230.8 μm), which reduces the organism’s natural food intake, brine shrimp eggs, due to a false sense of satiety, with potential impacts on coral health [13]. Table 3 shows the exposure of corals to MPs and the effects that appear, considering concentration and exposure time.

Fish
Ingestion of MPs by fish in marine environments can occur through contaminated bait, the water column, and also through bottom sediments [76]. The presence of MP particles mixed with sediment, as well as the presence of MPs suspended in the water column, leads to unintentional ingestion of MP particles by fish when they are foraging. In general, in fish, the amount of MP ingested increases with increasing fish size. This relationship exists until a certain maximum for the size of the plastic and the fish is reached, after which this relationship between the size of the fish and the amount of plastic ingested may no longer exist [77]. The size and amount of MPs that fish ingest are influenced by their feeding behavior. Omnivorous fish feed on both animal and plant food sources and consume a wider range of food, and therefore have a higher exposure to MP particles compared to herbivorous and carnivorous fish [76].
Results from analysis of the digestive tracts of various fish species confirm the presence of MP particles in the digestive tracts of these organisms. After ingestion, MPs accumulate in the digestive tract of fish, causing blockage of the digestive system and reduced feeding due to false satiety [12]. Ingestion of MPs can disrupt the structure and function of the digestive tract, leading to nutritional and growth problems in fish [78]. The color of MPs is a physical characteristic that influences the uptake of MPs in the marine food chain. Dark-colored MPs (black, blue, and green) are ingested by fish in greater amounts than light-colored MPs, likely due to their resemblance to real prey [79].
Yin et al. (2018) showed that exposure of Jacopever fish to PS MPs at a concentration of 106 particles/L reduced the weight gain rate by 65.4%, the growth rate by 65.9%, and the gross energy of fish by 9.5% [80]. Other effects of MPs in fish include inflammatory responses and immune system disorders [12]. Laboratory studies support the hypothesis that exposure to MPs results in toxicological effects in fish. The question here is whether toxicological effects also occur under environmental conditions [81]. This is because in the environment, organisms are exposed to a mixture of MPs, but most studies conducted to investigate the toxicity effects of MPs expose organisms to only one type of MP. Also, most studies used microbeads to investigate toxicity effects, while in marine environments, the predominant form of MPs is fibers. Also, concentrations of MPs in marine environments are very different from the concentrations that fish were exposed to in laboratory conditions. In the composition of plastics, there are some additives such as bisphenol A (BPA) and nonylphenol, which are used to improve the properties of the polymer. There is a possibility that the additives can leak from MPs and enter aquatic environments and fish [12].
Just as fibers and fragments are the most common forms of MPs in aquatic environments, they are also the most common forms of MPs detected in fish [82]. PP, polyester (PES), PE, and PS are the most abundant polymers found worldwide. These polymers were also the most abundant in the digestive tract of fish [83].
Alomar et al. (2017), who studied the presence of MP particles in the stomachs blackmouth catshark (Galeus melastomus) in the Mediterranean Sea, found high concentrations of MPs in the stomachs of this animal [82]. Most of these MPs remain in the fish’s stomach and intestines after ingestion. MP particles also have the ability to enter the circulatory or lymphatic system, where they can be distributed throughout the body. They also have the ability to stick to fish skin [84]. Figure 2 shows the entry of MPs into marine ecosystems due to human activities and its effects on marine organisms. Nerland et al. (2014) studied Japanese medaka (Oryzias latipes) over a long period of time, and their results showed that moderate concentrations (8 ngL−1 of PE of size <0.5 mm) of MPs in the sea (San Diego Bay, California) can have harmful but non-lethal effects on the fish [85]. In a study by Ramos et al. (2012) in a tropical river in northeastern Brazil, bottom-feeding fish (Gerreidae) in this river were found to be contaminated with MPs and their stomachs were severely affected by MP particles [86].
Batel et al. (2016) artificially simulated a food chain in an aquatic environment using brine shrimp (Artemia sp.) and zebrafish (Danio rerio) and concluded that MPs have the ability to transfer benzo(a)pyrene from brine shrimp (Artemia sp.) to zebrafish (D. rerio). This study demonstrated that fish may be exposed to contaminants attached to MP particles through ingestion of other marine organisms contaminated with MPs [87]. MPs may also carry pathogens and antibiotic resistance genes that may cause disease after being ingested by fish [88]. Table 4 shows the exposure of fish to MPs along with other pollutants and the effects that appear depending on the concentration and time of exposure.

Sea birds
In 2023, plasticosis was discovered in seabirds, a new disease caused solely by plastic. Birds identified as suffering from this disease had their digestive tracts injured by ingestion of plastic debris [93]. Following ingestion of small pieces of plastic, their digestive tracts become inflamed. Over time, persistent inflammation causes tissues to scar and deform, affecting digestion, growth, and survival [94].
Seabirds, including shearwaters, northern fulmars, petrels, albatrosses feed on the surface of the sea and ingest MP particles floating in the water. Ingested MPs accumulate and concentrate in the stomachs of seabirds. In studies, 30-35 percent of the plastics detected in the birds’ bodies were in the form of industrial pellets [68, 95, 96]. In the South Atlantic region, plastic pellets were also found in the digestive tracts of seabirds, which excreted the plastic along with their feces [97]. In a study by Vlietstra et al. (2002) on the short-tailed shearwater, which lives in the North Sea, MP particles were also found in the digestive tract of this bird [98].
Carlin et al. (2020) found MP particles in the digestive tracts of all species of birds of prey in Central Florida. The average total MPs in birds and per gram of bird digestive tract tissue were 11.9 and 0.3, respectively. In this study, the fiber form accounted for more than 86 percent of all plastics. Most of the fibers were clear or blue in color [99]. Seabirds, including Larus glaucescens, can remove MP particles from their digestive tracts through a process called regurgitation [100]. Studies have shown that younger northern fulmars (Fulmarus glacialis) ingest more MPs during the feeding process, which accumulate in their intestines. Ingesting MP particles causes hunger and loss of fitness [101].
Bilal et al. (2023) identified a total of 2033 MP particles in 20 samples of ducks (Anas platyrhynchos) from the Panjkora River. The mean abundance of MPs was 44.6±15.8 MPs in the crop and 57.05±18.7 MPs in the gizzard. Ducks are constantly exposed to MP pollution due to their proximity to polluted aquatic ecosystems and their feeding habits [102]. Figure 3 shows MPs contamination in seabirds and the transfer of MPs in the food chain.
Marine mammal and turtles
The effects of MPs have been studied in many large marine organisms, including harbor seals, sea turtles, polar bears, and whales [103]. Marine mammals can ingest MPs directly in the marine environment or indirectly through ingestion of smaller marine organisms that have consumed the MPs [6]. Ingested MPs may be excreted through pseudofaece or accumulate in the organism and move between tissues [4, 56].
The likelihood of ingesting MP particles and their accumulation in the stomach and intestines of whales is high, due to the high fat and lipid content of this animal. There are many reports of the death of stranded whales with intestines full of MP particles. Although there have been no reports of polar bears ingesting MPs, the likelihood of this creature ingesting MP particles is high [68]. In a study by Nerland et al. (2014), 60.5% of sea turtles showed accumulation of MP particles in their digestive tract [85].
Studies have shown that MP particles in gray seals living in a sanctuary where human activity and pollution were minimal, indicating the presence of MPs even in controlled environmental conditions [104]. As MP particles get smaller, they are more likely to be ingested by larger marine creatures. Whales (Megaptera novaeangliae) filter small food particles from large volumes of water, which means they ingest large amounts of MPs along with their food [105].
Examination of the gut contents of baleen whales shows that their diet often consisted of mesopelagic fish, cephalopods, and MPs, and MP particles were found throughout the whale’s digestive tract. It is likely that MPs in the whale’s digestive tract are eventually excreted. The stomachs of some baleen whales, including True’s beaked whale Mesoplodon mirus and Cuvier’s beaked whale Ziphius cavirostris, contained only plastic particles and no food was found in their stomachs [106]. The question now is whether whales are able to excrete ingested plastic particles and whether these plastic particles can cause blockages in their digestive tracts. It has yet to be determined whether some whales, such as filter feeding baleen whales, are able to excrete ingested plastic particles while some others, including beaked whales, are unable to excrete the plastic fragments they ingest. Additionally, there is considerable uncertainty about whether the size of ingested plastic can affect a whale’s ability to excrete plastic and whether size plays a role in gastrointestinal blockage [76]. Getting trapped in plastic waste and ingesting MPs causes acute and chronic damage and increases the pollutant load in the cetaceans, ultimately leading to their death [6].
Fiber is the most abundant form of MPs in the digestive tract of marine mammals [107]. MPs in various colors, such as black, white, green, and blue, have been reported in the digestive tract and feces of marine mammals [107, 108]. Among the polymers identified in marine mammals, nylon, cotton, PP, polyethersulfone, ethylene propylene, and polyester were the most abundant [109].
MPs have become a serious threat to marine mammals [110]. Consumption of MPs can reduce energy reserves, feeding capacity and reproduction. MPs can cause adverse changes in the physiology of the gastrointestinal tract and suppress the immune system. Vulnerability to diseases, oxidative stress and cytotoxicity are other effects of exposure to MPs [104, 111].
The nature of MPs determines the extent of bioaccumulation of pollutants in different tissues. MPs are likely to cross cell membranes and the blood-brain barrier in marine mammals, they can enter the bloodstream and concentrate in other vital internal organs such as the placenta, liver, and kidney [112].
MPs in marine environments provide a highly selective and flexible support platform for a variety of microbial communities, especially resistant pathogens [113]. Pathogens accumulated on MPs can travel long distances by waves, currents, and winds and be transmitted to different hosts, thereby causing disease outbreaks [114]. The concentration of antimicrobial-resistant pathogens on the surface of MPs is 100 to 5000 times higher than in the surrounding water [88]. Marine mammals are predators and may consume high concentrations of MPs through the food web, but to date, no studies have been conducted on whether pathogen-laden MPs can transmit pathogens and cause disease in marine mammals. Microorganisms attached to MPs use the adsorbed metals (e.g. cobalt, iron, zinc, manganese, copper) as micronutrients. Many pathogens require these metals for survival and pathogenicity [115]. MPs can disrupt the protective lung barriers of marine mammals, allowing opportunistic pathogens to penetrate deep into the lungs, leading to respiratory infections [114]. It was proven that marine mammals whose cause of death was infectious disease had much higher MPs among the factors leading to mortality [116].
Studies show that atmospheric deposition transports MP particles into the ocean surface air, so marine mammals and sea turtles that breathe ocean surface air are susceptible to inhaling MP particles. Inhaling airborne MPs can cause significant harm to sea turtles and marine mammals, as they inhale large amounts of air when breathing and hold their breath during prolonged diving in the seas and oceans, which increases the duration of exposure to MPs and other pollutants in the air [109]. Figure 4 shows the transport of MPs in the marine food web.
Human exposure to MPs through seafood diet
The accumulation of MPs in marine organisms consumed as human food leads to dangerous consequences for human health. Commercial marine organisms such as crab, oyster, fish, and sea cucumber ingest MP particles and transport them throughout the food web [68].
To identify the abundance of MPs in marine environments and marine organisms, appropriate indicator species are needed to assess MP pollution, exposure to MPs, and environmental and human risks [117]. Mussels (Mytilus spp.) are one of the marine indicator organisms for the assessment of the above. They are able to tolerate different environmental conditions and are widely distributed in marine environments. Mussels are also filter feeders and are directly exposed to MP particles, so the concentration of MPs in mussel tissues has a close relationship with the concentration of MPs in marine environments. Mollusks are the most important route of human exposure to MPs through the marine diet, as these organisms are fully consumed by humans, making them a good indicator of MP contamination and the safety of seafood consumed by humans [118, 119].
Research by Lia et al. (2018) shows that mussels collected from different areas, including coastal waters and supermarkets, are contaminated with MP particles and predicted that consumers eat 70 MPs in 100 grams of mussels [120]. MPs were also detected in sardines and sprats cans [121] and the organs (e.g. stomach contents and liver) of fish Squalius cephalus [122]. It has also been reported that consuming Mud carp (Cirrhinus molitorella) and Bighead carp (Hypophthalmichthys nobilis) have the potential risk of accumulating phthalate esters along gastrointestinal tract [123].
Young fish, arthropods, and mollusks that are prey to shrimp may be contaminated with MP particles. These MP particles accumulate in the digestive tract of shrimp after ingestion of the aforementioned organisms. Given that shrimp are consumed whole by humans without removing the digestive tract, consumption of shrimp is a potential route for human exposure to MPs through seafood [119].
Human exposure to MP particles results in physical and chemical toxic effects. The sensitivity of the exposed individual and the type of MP particles are factors influencing the development of adverse effects. The physical effects may have different concerning impacts, including enhanced toxicity, cell damage, inflammatory response, and oxidative stress [10]. Since the immune system cannot eliminate MPs particles, these particles lead to chronic inflammation and an increased risk of neoplasia [124]. The chemical effects of MP particles on humans are caused either by the absorption of pollutants from the environment (PAH, PCB, and toxic metals) into MPs or by the chemicals used in the production of plastics [60]. Among these chemicals, BPA and phthalates cause endocrine disruption, neurotoxic effects, and cancer in humans [125]. Most of the information on MPs in the marine food web is related to their presence in the digestive tract of seafood, while in many cases the digestive tract of marine organisms is discarded before consumption by humans [126]. Also, when assessing the risk of MPs to human health, it is necessary to consider the risk of pathogenic microorganisms adsorbed to MP particles, as there is a possibility of enhanced growth and colony formation of pathogens in the MP substrate [119].
Conclusion
Over the past few years, plastic waste has played an undeniable role in causing pollution in terrestrial, aerial, and aquatic ecosystems. They are known to be a major environmental problem, especially in aquatic environments. Plastic polymers degrade and fragment in aquatic environments as a result of environmental processes (thermal degradation, sunlight, and biofilm growth and oxidation), and those that reach a size of <5 mm are called MPs. The presence of MPs in marine environments and their transfer through the marine food chain has endangered a wide range of marine organisms, including plankton, bivalves, corals, fish, seabirds, and marine mammals. Exposure of marine organisms to MPs can lead to weight loss, reduced growth, reduced reproduction, changes in feeding behavior, changes in enzyme activity, initiation of stress response, and inflammatory response. Atmospheric deposition transports MP particles into the ocean surface air, so marine mammals and sea turtles that breathe ocean surface air are susceptible to inhaling MP particles in addition to ingesting them. The accumulation of MPs in marine organisms consumed as human food leads to dangerous consequences for human health. Commercial marine organisms such as crab, oyster, fish, and sea cucumber ingest MP particles and transport them throughout the food web. Human exposure to MP particles results in physical and chemical toxic effects.
Ethical Considerations
Compliance with ethical guidelines
There were no ethical considerations to be considered in this work.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
All authors equally contributed to preparing this article.
Conflict of interest
The authors declared no conflict of interest
Acknowledgements
The authors are most grateful to the Department of Environmental Health Engineering, School of Public Health, Guilan University of Medical Sciences, Rasht, Iran, for their collaboration in this research.
References
- Ugwu K, Herrera A, Gómez M. Microplastics in marine biota: A review. Mar Pollut Bull. 2021; 169:112540. [DOI:10.1016/j.marpolbul.2021.112540] [PMID]
- Akbari A, Jaafari J. Entry pathway and potential impacts of microplastics in air, water, soil and human health: A review. Caspian J Health Res. 2024; 9(4):181-98. [DOI:10.32598/CJHR.9.4.254.2]
- Anbumani S, Kakkar P. Ecotoxicological effects of microplastics on biota: A review. Environ Sci Pollut Res Int. 2018; 25(15):14373-96. [DOI:10.1007/s11356-018-1999-x] [PMID]
- Thompson RC, Olsen Y, Mitchell RP, Davis A, Rowland SJ, John AW, et al. Lost at sea: Where is all the plastic? Science. 2004; 304(5672):838. [DOI:10.1126/science.1094559] [PMID]
- Akbari A, Taghavi K, Jaafari J. Sources of microplastics in the environment and human exposure routes: A review. Caspian J Health Res. 2024; 9(3):163-76. [DOI:10.32598/CJHR.9.3.254.1]
- Guzzetti E, Sureda A, Tejada S, Faggio C. Microplastic in marine organism: Environmental and toxicological effects. Environ Toxicol Pharmacol. 2018; 64:164-71. [DOI:10.1016/j.etap.2018.10.009] [PMID]
- Besseling E, Wang B, Lurling M, Koelmans AA. Nanoplastic affects growth of S. obliquus and reproduction of D. magna. Environ Sci Technol. 2014; 48(20):12336-43. [DOI:10.1021/es503001d] [PMID]
- Majer AP, Vedolin MC, Turra A. Plastic pellets as oviposition site and means of dispersal for the ocean-skater insect Halobates. Mar Pollut Bull. 2012; 64(6):1143-7. [DOI:10.1016/j.marpolbul.2012.03.029] [PMID]
- Pennino MG, Bachiller E, Lloret-Lloret E, Albo-Puigserver M, Esteban A, Jadaud A, et al. Ingestion of microplastics and occurrence of parasite association in Mediterranean anchovy and sardine. Mar Pollut Bull. 2020; 158:111399. [DOI:10.1016/j.marpolbul.2020.111399] [PMID]
- Smith M, Love DC, Rochman CM, Neff RA. Microplastics in seafood and the implications for human health. Curr Environ Health Rep. 2018; 5(3):375-86. [DOI:10.1007/s40572-018-0206-z] [PMID]
- Zantis LJ, Carroll EL, Nelms SE, Bosker T. Marine mammals and microplastics: A systematic review and call for standardisation. Environ Pollut. 2021; 269:116142. [DOI:10.1016/j.envpol.2020.116142] [PMID]
- Wang W, Ge J, Yu X. Bioavailability and toxicity of microplastics to fish species: A review. Ecotoxicol Environ Saf. 2020; 189:109913. [DOI:10.1016/j.ecoenv.2019.109913] [PMID]
- Huang W, Chen M, Song B, Deng J, Shen M, Chen Q, et al. Microplastics in the coral reefs and their potential impacts on corals: A mini-review. Sci Total Environ. 2021; 762:143112. [DOI:10.1016/j.scitotenv.2020.143112] [PMID]
- Bom FC, Sá F. Concentration of microplastics in bivalves of the environment: A systematic review. Environ Monit Assess. 2021; 193(12):846. [DOI:10.1007/s10661-021-09639-1] [PMID]
- De-la-Torre GE, Dioses-Salinas DC, Castro JM, Antay R, Fernández NY, Espinoza-Morriberón D, et al. Abundance and distribution of microplastics on sandy beaches of Lima, Peru. Mar Pollut Bull. 2020; 151:110877. [DOI:10.1016/j.marpolbul.2019.110877] [PMID]
- Karlsson TM, Kärrman A, Rotander A, Hassellöv M. Comparison between manta trawl and in situ pump filtration methods, and guidance for visual identification of microplastics in surface waters. Environ Sci Pollut Res Int. 2020; 27(5):5559-71. [DOI:10.1007/s11356-019-07274-5] [PMID]
- Lebreton LCM, van der Zwet J, Damsteeg JW, Slat B, Andrady A, Reisser J. River plastic emissions to the world’s oceans. Nat Commun. 2017; 8:15611. [DOI:10.1038/ncomms15611] [PMID]
- Horton AA, Svendsen C, Williams RJ, Spurgeon DJ, Lahive E. Large microplastic particles in sediments of tributaries of the River Thames, UK-Abundance, sources and methods for effective quantification. Mar Pollut Bull. 2017; 114(1):218-26. [DOI:10.1016/j.marpolbul.2016.09.004] [PMID]
- Liao B, Wang J, Xiao B, Yang X, Xie Z, Li D, et al. Effects of acute microplastic exposure on physiological parameters in Tubastrea aurea corals. Mar Pollut Bull. 2021; 165:112173. [DOI:10.1016/j.marpolbul.2021.112173] [PMID]
- Jambeck JR, Geyer R, Wilcox C, Siegler TR, Perryman M, Andrady A, et al. Plastic waste inputs from land into the ocean. Science. 2015; 347(6223):768-71. [DOI:10.1126/science.1260352] [PMID]
- Andrady AL. The plastic in microplastics: A review. Mar Pollut Bull. 2017; 119(1):12-22. [DOI:10.1016/j.marpolbul.2017.01.082] [PMID]
- Rincon-Rubio L, Fayolle B, Audouin L, Verdu J. A general solution of the closed-loop kinetic scheme for the thermal oxidation of polypropylene. Polym Degrad Stab. 2001; 74(1):177-88. [DOI:10.1016/S0141-3910(01)00154-9]
- Yan M, Nie H, Xu K, He Y, Hu Y, Huang Y, et al. Microplastic abundance, distribution and composition in the Pearl River along Guangzhou city and Pearl River estuary, China. Chemosphere. 2019; 217:879-86. [DOI:10.1016/j.chemosphere.2018.11.093] [PMID]
- Carr SA, Liu J, Tesoro AG. Transport and fate of microplastic particles in wastewater treatment plants. Water Res. 2016; 91:174-82. [DOI:10.1016/j.watres.2016.01.002] [PMID]
- Barnes DK, Galgani F, Thompson RC, Barlaz M. Accumulation and fragmentation of plastic debris in global environments. Philos Trans R Soc Lond B Biol Sci. 2009; 364(1526):1985-98. [DOI:10.1098/rstb.2008.0205] [PMID]
- Galloway TS, Cole M, Lewis C. Interactions of microplastic debris throughout the marine ecosystem. Nat Ecol Evol. 2017; 1(5):116. [DOI:10.1038/s41559-017-0116] [PMID]
- Rodrigues SM, Elliott M, Almeida CMR, Ramos S. Microplastics and plankton: Knowledge from laboratory and field studies to distinguish contamination from pollution. J Hazard Mater. 2021; 417:126057. [DOI:10.1016/j.jhazmat.2021.126057] [PMID]
- Setälä O, Fleming-Lehtinen V, Lehtiniemi M. Ingestion and transfer of microplastics in the planktonic food web. Environ Pollut. 2014; 185:77-83. [DOI:10.1016/j.envpol.2013.10.013] [PMID]
- Murray F, Cowie PR. Plastic contamination in the decapod crustacean Nephrops norvegicus (Linnaeus, 1758). Mar Pollut Bull. 2011; 62(6):1207-17. [DOI:10.1016/j.marpolbul.2011.03.032] [PMID]
- Zhang F, Wang X, Xu J, Zhu L, Peng G, Xu P, et al. Food-web transfer of microplastics between wild caught fish and crustaceans in East China Sea. Mar Pollut Bull. 2019; 146:173-82. [DOI:10.1016/j.marpolbul.2019.05.061] [PMID]
- Colomer J, Müller MF, Barcelona A, Serra T. Mediated food and hydrodynamics on the ingestion of microplastics by Daphnia magna. Environ Pollut. 2019; 251:434-41. [DOI:10.1016/j.envpol.2019.05.034] [PMID]
- Murphy F, Quinn B. The effects of microplastic on freshwater Hydra attenuata feeding, morphology & reproduction. Environ Pollut. 2018; 234:487-94. [DOI:10.1016/j.envpol.2017.11.029] [PMID]
- Gambardella C, Morgana S, Ferrando S, Bramini M, Piazza V, Costa E, et al. Effects of polystyrene microbeads in marine planktonic crustaceans. Ecotoxicol Environ Saf. 2017;145:250-7. [DOI:10.1016/j.ecoenv.2017.07.036] [PMID]
- Tang J, Wang X, Yin J, Han Y, Yang J, Lu X, et al. Molecular characterization of thioredoxin reductase in waterflea Daphnia magna and its expression regulation by polystyrene microplastics. Aquat Toxicol. 2019; 208:90-7. [DOI:10.1016/j.aquatox.2019.01.001] [PMID]
- Cole M, Lindeque P, Fileman E, Halsband C, Goodhead R, Moger J, et al. Microplastic ingestion by zooplankton. Environ Sci Technol. 2013; 47(12):6646-55. [DOI:10.1021/es400663f] [PMID]
- Watts AJ, Lewis C, Goodhead RM, Beckett SJ, Moger J, Tyler CR, et al. Uptake and retention of microplastics by the shore crab Carcinus maenas. Environ Sci Technol. 2014; 48(15):8823-30. [DOI:10.1021/es501090e] [PMID]
- Davarpanah E, Guilhermino L. Single and combined effects of microplastics and copper on the population growth of the marine microalgae Tetraselmis chuii. Estuar Coast Shelf Sci. 2015; 167:269-75. [DOI:10.1016/j.ecss.2015.07.023]
- Long M, Moriceau B, Gallinari M, Lambert C, Huvet A, Raffray J, et al. Interactions between microplastics and phytoplankton aggregates: impact on their respective fates. Mar Chem. 2015; 175:39-46. [DOI:10.1016/j.marchem.2015.04.003]
- Steer M, Cole M, Thompson RC, Lindeque PK. Microplastic ingestion in fish larvae in the western English Channel. Environ Pollut. 2017; 226:250-9. [DOI:10.1016/j.envpol.2017.03.062] [PMID]
- De Lucia GA, Vianello A, Camedda A, Vani D, Tomassetti P, Coppa S, et al. Sea water contamination in the vicinity of the Italian minor islands caused by microplastic pollution. Water. 2018; 10(8):1108. [DOI:10.3390/w10081108]
- Lusher AL, Tirelli V, O’Connor I, Officer R. Microplastics in Arctic polar waters: The first reported values of particles in surface and sub-surface samples. Sci Rep. 2015; 5:14947. [DOI:10.1038/srep14947] [PMID]
- Payton TG, Beckingham BA, Dustan P. Microplastic exposure to zooplankton at tidal fronts in Charleston Harbor, SC USA. Estuar Coast Shelf Sci. 2020; 232:106510. [DOI:10.1016/j.ecss.2019.106510]
- Kang JH, Kwon OY, Shim WJ. Potential threat of microplastics to zooplanktivores in the surface waters of the Southern Sea of Korea. Arch Environ Contam Toxicol. 2015; 69(3):340-51. [DOI:10.1007/s00244-015-0210-3] [PMID]
- Alfonso MB, Arias AH, Piccolo MC. Microplastics integrating the zooplanktonic fraction in a saline lake of Argentina: Influence of water management. Environ Monit Assess. 2020; 192(2):117. [DOI:10.1007/s10661-020-8080-1] [PMID]
- Hitchcock JN, Mitrovic SM. Microplastic pollution in estuaries across a gradient of human impact. Environ Pollut. 2019; 247:457-66. [DOI:10.1016/j.envpol.2019.01.069] [PMID]
- Di Mauro R, Kupchik MJ, Benfield MC. Abundant plankton-sized microplastic particles in shelf waters of the northern Gulf of Mexico. Environ Pollut. 2017; 230:798-809.[DOI:10.1016/j.envpol.2017.07.030] [PMID]
- Zhang F, Man YB, Mo WY, Man KY, Wong MH. Direct and indirect effects of microplastics on bivalves, with a focus on edible species: A mini-review. Crit Rev Environ Sci Technol. 2020; 50(20):2109-43. [DOI:10.1080/10643389.2019.1700752]
- Ward JE, Shumway SE. Separating the grain from the chaff: Particle selection in suspension-and deposit-feeding bivalves. J Exp Mar Bio Ecol. 2004; 300(1-2):83-130. [DOI:10.1016/j.jembe.2004.03.002]
- Naidu S, Ranga Rao V, Ramu K. Microplastics in the benthic invertebrates from the coastal waters of Kochi, Southeastern Arabian Sea. Environ Geochem Health. 2018; 40(4):1377-83. [DOI:10.1007/s10653-017-0062-z] [PMID]
- Renzi M, Guerranti C, Blašković A. Microplastic contents from maricultured and natural mussels. Mar Pollut Bull. 2018; 131(Pt A):248-51. [DOI:10.1016/j.marpolbul.2018.04.035] [PMID]
- Qu X, Su L, Li H, Liang M, Shi H. Assessing the relationship between the abundance and properties of microplastics in water and in mussels. Sci Total Environ. 2018; 621:679-86. [DOI:10.1016/j.scitotenv.2017.11.284] [PMID]
- Li J, Yang D, Li L, Jabeen K, Shi H. Microplastics in commercial bivalves from China. Environ Pollut. 2015; 207:190-5. [DOI:10.1016/j.envpol.2015.09.018] [PMID]
- Wegner A, Besseling E, Foekema EM, Kamermans P, Koelmans AA. Effects of nanopolystyrene on the feeding behavior of the blue mussel (Mytilus edulis L.). Environ Toxicol Chem. 2012; 31(11):2490-7. [DOI:10.1002/etc.1984] [PMID]
- Rist SE, Assidqi K, Zamani NP, Appel D, Perschke M, Huhn M, et al. Suspended micro-sized PVC particles impair the performance and decrease survival in the Asian green mussel Perna viridis. Mar Pollut Bull. 2016; 111(1-2):213-20. [DOI:10.1016/j.marpolbul.2016.07.006] [PMID]
- Guilhermino L, Vieira LR, Ribeiro D, Tavares AS, Cardoso V, Alves A, et al. Uptake and effects of the antimicrobial florfenicol, microplastics and their mixtures on freshwater exotic invasive bivalve Corbicula fluminea. Sci Total Environ. 2018; 622-623:1131-42. [DOI:10.1016/j.scitotenv.2017.12.020] [PMID]
- Avio CG, Gorbi S, Milan M, Benedetti M, Fattorini D, d’Errico G, et al. Pollutants bioavailability and toxicological risk from microplastics to marine mussels. Environ Pollut. 2015; 198:211-22. [DOI:10.1016/j.envpol.2014.12.021] [PMID]
- Sussarellu R, Suquet M, Thomas Y, Lambert C, Fabioux C, Pernet ME, et al. Oyster reproduction is affected by exposure to polystyrene microplastics. Proc Natl Acad Sci U S A. 2016; 113(9):2430-5. [DOI:10.1073/pnas.1519019113] [PMID]
- Van Cauwenberghe L, Janssen CR. Microplastics in bivalves cultured for human consumption. Environ Pollut. 2014; 193:65-70. [DOI:10.1016/j.envpol.2014.06.010] [PMID]
- Xu XY, Lee WT, Chan AKY, Lo HS, Shin PKS, Cheung SG. Microplastic ingestion reduces energy intake in the clam Atactodea striata. Mar Pollut Bull. 2017; 124(2):798-802. [DOI:10.1016/j.marpolbul.2016.12.027] [PMID]
- Barboza LGA, Dick Vethaak A, Lavorante BRBO, Lundebye AK, Guilhermino L. Marine microplastic debris: An emerging issue for food security, food safety and human health. Mar Pollut Bull. 2018; 133:336-48. [DOI:10.1016/j.marpolbul.2018.05.047] [PMID]
- Magni S, Gagné F, André C, Della Torre C, Auclair J, Hanana H, et al. Evaluation of uptake and chronic toxicity of virgin polystyrene microbeads in freshwater zebra mussel Dreissena polymorpha (Mollusca: Bivalvia). Sci Total Environ. 2018; 631-632:778-88. [DOI:10.1016/j.scitotenv.2018.03.075] [PMID]
- Revel M, Lagarde F, Perrein-Ettajani H, Bruneau M, Akcha F, Sussarellu R, et al. Tissue-specific biomarker responses in the blue mussel Mytilus spp. exposed to a mixture of microplastics at environmentally relevant concentrations. Front Environ Sci. 2019; 7:33. [DOI:10.3389/fenvs.2019.00033]
- Green DS, Boots B, O’Connor NE, Thompson R. Microplastics affect the ecological functioning of an important biogenic habitat. Environ Sci Technol. 2017; 51(1):68-77. [DOI:10.1021/acs.est.6b04496] [PMID]
- John J, Nandhini AR, Velayudhaperumal Chellam P, Sillanpää M. Microplastics in mangroves and coral reef ecosystems: A review. Environ Chem Lett. 2022; 20(1):397-416. [DOI:10.1007/s10311-021-01326-4] [PMID]
- Yap HT. Coral reef ecosystems. In: Orcutt J, editor. Earth system monitoring. New York: Springer; 2013.[DOI:10.1007/978-1-4614-5684-1_5]
- Ding J, Jiang F, Li J, Wang Z, Sun C, Wang Z, et al. Microplastics in the coral reef systems from Xisha Islands of South China Sea. Environ Sci Technol. 2019; 53(14):8036-46. [DOI:10.1021/acs.est.9b01452] [PMID]
- Imhof HK, Sigl R, Brauer E, Feyl S, Giesemann P, Klink S, et al. Spatial and temporal variation of macro-, meso-and microplastic abundance on a remote coral island of the Maldives, Indian Ocean. Mar Pollut Bull. 2017; 116(1-2):340-7. [DOI:10.1016/j.marpolbul.2017.01.010] [PMID]
- Sharma S, Chatterjee S. Microplastic pollution, a threat to marine ecosystem and human health: A short review. Environ Sci Pollut Res Int. 2017; 24(27):21530-247. [DOI:10.1007/s11356-017-9910-8] [PMID]
- Hall N, Berry K, Rintoul L, Hoogenboom M. Microplastic ingestion by scleractinian corals. Mar Biol. 2015; 162:725-32. [DOI:10.1007/s00227-015-2619-7]
- Corona E, Martin C, Marasco R, Duarte CM. Passive and active removal of marine microplastics by a mushroom coral (Danafungia scruposa). Front Mar Sci. 2020; 7:128. [DOI:10.3389/fmars.2020.00128]
- Mendrik FM, Henry TB, Burdett H, Hackney CR, Waller C, Parsons DR, et al. Species-specific impact of microplastics on coral physiology. Environ Pollut. 2021; 269:116238. [DOI:10.1016/j.envpol.2020.116238] [PMID]
- Hankins C, Moso E, Lasseigne D. Microplastics impair growth in two atlantic scleractinian coral species, Pseudodiploria clivosa and Acropora cervicornis. Environ Pollut. 2021; 275:116649. [DOI:10.1016/j.envpol.2021.116649] [PMID]
- Cordova MR, Hadi TA, Prayudha B. Occurrence and abundance of microplastics in coral reef sediment: A case study in Sekotong, Lombok-Indonesia. AES Bioflux. 2018; 10(1):23-9.[Link]
- Santana MF, Dawson AL, Motti CA, Van Herwerden L, Lefevre C, Kroon FJ. Ingestion and depuration of microplastics by a planktivorous coral reef fish, Pomacentrus amboinensis. Front Environ Sci. 2021; 9:641135. [DOI:10.3389/fenvs.2021.641135]
- Lanctôt CM, Bednarz VN, Melvin S, Jacob H, Oberhaensli F, Swarzenski PW, et al. Physiological stress response of the scleractinian coral Stylophora pistillata exposed to polyethylene microplastics. Environ Pollut. 2020; 263(Pt A):114559. [DOI:10.1016/j.envpol.2020.114559] [PMID]
- Egbeocha CO, Malek S, Emenike CU, Milow P. Feasting on microplastics: Ingestion by and effects on marine organisms. Aquat Bio. 2018; 27:93-106. [DOI:10.3354/ab00701]
- Boerger CM, Lattin GL, Moore SL, Moore CJ. Plastic ingestion by planktivorous fishes in the North Pacific Central Gyre. Mar Pollut Bull. 2010; 60(12):2275-8. [DOI:10.1016/j.marpolbul.2010.08.007] [PMID]
- Jabeen K, Li B, Chen Q, Su L, Wu C, Hollert H, et al. Effects of virgin microplastics on goldfish (Carassius auratus). Chemosphere. 2018; 213:323-32. [DOI:10.1016/j.chemosphere.2018.09.031] [PMID]
- Ma H, Pu S, Liu S, Bai Y, Mandal S, Xing B. Microplastics in aquatic environments: Toxicity to trigger ecological consequences. Environ Pollut. 2020; 261:114089. [DOI:10.1016/j.envpol.2020.114089] [PMID]
- Yin L, Chen B, Xia B, Shi X, Qu K. Polystyrene microplastics alter the behavior, energy reserve and nutritional composition of marine jacopever (Sebastes schlegelii). J Hazard Mater. 2018; 360:97-105. [DOI:10.1016/j.jhazmat.2018.07.110] [PMID]
- Karami A. Gaps in aquatic toxicological studies of microplastics. Chemosphere. 2017; 184:841-8. [DOI:10.1016/j.chemosphere.2017.06.048] [PMID]
- Alomar C, Deudero S. Evidence of microplastic ingestion in the shark Galeus melastomus Rafinesque, 1810 in the continental shelf off the western Mediterranean Sea. Environ Pollut. 2017; 223:223-9. [DOI:10.1016/j.envpol.2017.01.015] [PMID]
- Rummel CD, Löder MG, Fricke NF, Lang T, Griebeler EM, Janke M, et al. Plastic ingestion by pelagic and demersal fish from the North Sea and Baltic Sea. Mar Pollut Bull. 2016; 102(1):134-41. [DOI:10.1016/j.marpolbul.2015.11.043] [PMID]
- Wright SL, Kelly FJ. Plastic and human health: A micro issue? Environ Sci Technol. 2017; 51(12):6634-47. [DOI:10.1021/acs.est.7b00423] [PMID]
- Nerland IL, Halsband C, Allan I, Thomas KV. Microplastics in marine environments: Occurrence, distribution and effects. Oslo: Norwegian Institute for Water Research; 2014. [Link]
- Ramos JA, Barletta M, Costa MF. Ingestion of nylon threads by Gerreidae while using a tropical estuary as foraging grounds. Aquat Biol. 2012; 17(1):29-34. [DOI:10.3354/ab00461]
- Batel A, Linti F, Scherer M, Erdinger L, Braunbeck T. Transfer of benzo [a] pyrene from microplastics to Artemia nauplii and further to zebrafish via a trophic food web experiment: CYP1A induction and visual tracking of persistent organic pollutants. Environ Toxicol Chem. 2016; 35(7):1656-66. [DOI:10.1002/etc.3361] [PMID]
- Yang Y, Liu G, Song W, Ye C, Lin H, Li Z, et al. Plastics in the marine environment are reservoirs for antibiotic and metal resistance genes. Environ Int. 2019; 123:79-86. [DOI:10.1016/j.envint.2018.11.061] [PMID]
- Sleight VA, Bakir A, Thompson RC, Henry TB. Assessment of microplastic-sorbed contaminant bioavailability through analysis of biomarker gene expression in larval zebrafish. Mar Pollut Bull. 2017; 116(1-2):291-7. [DOI:10.1016/j.marpolbul.2016.12.055] [PMID]
- Qu H, Ma R, Wang B, Yang J, Duan L, Yu G. Enantiospecific toxicity, distribution and bioaccumulation of chiral antidepressant venlafaxine and its metabolite in loach (Misgurnus anguillicaudatus) co-exposed to microplastic and the drugs. J Hazard Mater. 2019; 370:203-11. [DOI:10.1016/j.jhazmat.2018.04.041] [PMID]
- Nematdoost Haghi B, Banaee M. Effects of micro-plastic particles on paraquat toxicity to common carp (Cyprinus carpio): Biochemical changes. Int J Environ Sci Technol. 2017; 14(3):521-30. [DOI:10.1007/s13762-016-1171-4]
- Romano N, Ashikin M, Teh JC, Syukri F, Karami A. Effects of pristine polyvinyl chloride fragments on whole body histology and protease activity in silver barb Barbodes gonionotus fry. Environ Pollut. 2018; 237:1106-11. [DOI:10.1016/j.envpol.2017.11.040] [PMID]
- Horton H. New disease caused by plastics discovered in seabirds [internet]. 2023. [Updated 10 December 2024]. Available from: [Link]
- Ashworth J. New disease caused solely by plastics discovered in seabirds. 2023. [Updated 10 December 2024]. Available from: [Link]
- Robards MD, Piatt JF, Wohl KD. Increasing frequency of plastic particles ingested by seabirds in the subarctic North Pacific. Mar Pollut Bull. 1995; 30(2):151-7. [DOI:10.1016/0025-326X(94)00121-O]
- Blight LK, Burger AE. Occurrence of plastic particles in seabirds from the eastern North Pacific. Mar Pollut Bull. 1997; 34(5):323-5. [DOI:10.1016/S0025-326X(96)00095-1]
- Ryan PG. Seabirds indicate changes in the composition of plastic litter in the Atlantic and south-western Indian Oceans. Mar Pollut Bull. 2008; 56(8):1406-9. [DOI:10.1016/j.marpolbul.2008.05.004] [PMID]
- Vlietstra LS, Parga JA. Long-term changes in the type, but not amount, of ingested plastic particles in short-tailed shearwaters in the southeastern Bering Sea. Mar Pollut Bull. 2002; 44(9):945-55. [DOI:10.1016/S0025-326X(02)00130-3] [PMID]
- Carlin J, Craig C, Little S, Donnelly M, Fox D, Zhai L, et al. Microplastic accumulation in the gastrointestinal tracts in birds of prey in central Florida, USA. Environ Pollut. 2020; 264:114633. [DOI:10.1016/j.envpol.2020.114633] [PMID]
- Lindborg VA, Ledbetter JF, Walat JM, Moffett C. Plastic consumption and diet of Glaucous-winged Gulls (Larus glaucescens). Mar Pollut Bull. 2012; 64(11):2351-6.[DOI:10.1016/j.marpolbul.2012.08.020] [PMID]
- Kühn S, van Franeker JA. Plastic ingestion by the northern fulmar (Fulmarus glacialis) in Iceland. Mar Pollut Bull. 2012; 64(6):1252-4. [DOI:10.1016/j.marpolbul.2012.02.027] [PMID]
- Bilal M, Yaqub A, Hassan HU, Akhtar S, Rafiq N, Ali Shah MI, et al. Microplastic quantification in aquatic birds: Biomonitoring the environmental health of the Panjkora River freshwater ecosystem in Pakistan. Toxics. 2023; 11(12):972. [DOI:10.3390/toxics11120972] [PMID]
- Derraik JG. The pollution of the marine environment by plastic debris: A review. Mar Pollut Bull. 2002; 44(9):842-52. [DOI:10.1016/S0025-326X(02)00220-5] [PMID]
- Nabi G, Ahmad S, Ullah S, Zada S, Sarfraz M, Guo X, et al. The adverse health effects of increasing microplastic pollution on aquatic mammals. J King Saud Univ Sci. 2022; 34(4):102006. [DOI:10.1016/j.jksus.2022.102006]
- Fossi MC, Panti C, Guerranti C, Coppola D, Giannetti M, Marsili L, et al. Are baleen whales exposed to the threat of microplastics? A case study of the Mediterranean fin whale (Balaenoptera physalus). Mar Pollut Bull. 2012; 64(11):2374-9. [DOI:10.1016/j.marpolbul.2012.08.013] [PMID]
- Souza S, Siciliano S, Cuenca S, Sanctis B. A True’s beaked whale (Mesoplodon mirus) on the coast of Brazil: adding a new beaked whale species to the Western Tropical Atlantic and South America. Lat Am J Aquat Mammals. 2005; 4(2):129-36. [DOI:10.5597/lajam00077]
- Xiong X, Chen X, Zhang K, Mei Z, Hao Y, Zheng J, et al. Microplastics in the intestinal tracts of East Asian finless porpoises (Neophocaena asiaeorientalis sunameri) from Yellow Sea and Bohai Sea of China. Mar Pollut Bull. 2018; 136:55-60. [DOI:10.1016/j.marpolbul.2018.09.006] [PMID]
- Hernandez-Gonzalez A, Saavedra C, Gago J, Covelo P, Santos MB, Pierce GJ. Microplastics in the stomach contents of common dolphin (Delphinus delphis) stranded on the Galician coasts (NW Spain, 2005-2010). Mar Pollut Bull. 2018; 137:526-32. [DOI:10.1016/j.marpolbul.2018.10.026] [PMID]
- Meaza I, Toyoda JH, Wise JP Sr. Microplastics in sea turtles, marine mammals and humans: A one environmental health perspective. Front Environ Sci. 2020; 8:575614. [DOI:10.3389/fenvs.2020.575614] [PMID]
- De Sá LC, Oliveira M, Ribeiro F, Rocha TL, Futter MN. Studies of the effects of microplastics on aquatic organisms: What do we know and where should we focus our efforts in the future? Sci Total Environ. 2018; 645:1029-39. [DOI:10.1016/j.scitotenv.2018.07.207] [PMID]
- Hall AJ, Hugunin K, Deaville R, Law RJ, Allchin CR, Jepson PD. The risk of infection from polychlorinated biphenyl exposure in the harbor porpoise (Phocoena phocoena): A case-control approach. Environ Health Perspect. 2006; 114(5):704-11. [DOI:10.1289/ehp.8222] [PMID]
- Prietl B, Meindl C, Roblegg E, Pieber TR, Lanzer G, Fröhlich E. Nano-sized and micro-sized polystyrene particles affect phagocyte function. Cell Biol Toxicol. 2014; 30(1):1-16. [DOI:10.1007/s10565-013-9265-y] [PMID]
- Gkoutselis G, Rohrbach S, Harjes J, Obst M, Brachmann A, Horn MA, et al. Microplastics accumulate fungal pathogens in terrestrial ecosystems. Sci Rep. 2021; 11(1):13214.[DOI:10.1038/s41598-021-92405-7] [PMID]
- Bowley J, Baker-Austin C, Porter A, Hartnell R, Lewis C. Oceanic hitchhikers-assessing pathogen risks from marine microplastic. Trends Microbiol. 2021; 29(2):107-16.[DOI:10.1016/j.tim.2020.06.011] [PMID]
- Begg SL. The role of metal ions in the virulence and viability of bacterial pathogens. Biochem Soc Trans. 2019; 47(1):77-87. [DOI:10.1042/BST20180275] [PMID]
- Nelms SE, Barnett J, Brownlow A, Davison N, Deaville R, Galloway TS, et al. Microplastics in marine mammals stranded around the British coast: Ubiquitous but transitory? Sci Rep. 2019; 9(1):1075. [DOI:10.1038/s41598-018-37428-3] [PMID]
- Avio CG, Gorbi S, Regoli F. Experimental development of a new protocol for extraction and characterization of microplastics in fish tissues: First observations in commercial species from Adriatic Sea. Mar Environ Res. 2015; 111:18-26 [DOI:10.1016/j.marenvres.2015.06.014] [PMID]
- Li J, Lusher AL, Rotchell JM, Deudero S, Turra A, Bråte ILN, et al. Using mussel as a global bioindicator of coastal microplastic pollution. Environ Pollut. 2019; 244:522-33. [DOI:10.1016/j.envpol.2018.10.032] [PMID]
- Mercogliano R, Avio CG, Regoli F, Anastasio A, Colavita G, Santonicola S. Occurrence of microplastics in commercial seafood under the perspective of the human food chain. A review. J Agric Food Chem. 2020; 68(19):5296-301. [DOI:10.1021/acs.jafc.0c01209] [PMID]
- Lia J, Greenc C, Reynoldsd A, Shia H, Rotchellb JM. Microplastics in mussels sampled from coastal waters and supermarkets in the United Kingdom. Environ Pollut. 2018: 241:35-44. [DOI: 10.1016/j.envpol.2018.05.038] [PMID]
- Karami A, Golieskardi A, Choo CK, Larat V, Karbalaei S, Salamatinia B. Microplastic and mesoplastic contamination in canned sardines and sprats. Sci Total Environ. 2018; 612:1380-6. [DOI:10.1016/j.scitotenv.2017.09.005] [PMID]
- Collard F, Gasperi J, Gilbert B, Eppe G, Azimi S, Rocher V, et al. Anthropogenic particles in the stomach contents and liver of the freshwater fish Squalius cephalus. Sci Total Environ. 2018 ;643:1257-64. [DOI:10.1016/j.scitotenv.2018.06.313] [PMID]
- Cheng Z, Nie XP, Wang HS, Wong MH. Risk assessments of human exposure to bioaccessible phthalate esters through market fish consumption. Environ Int. 2013; 57-58:75-80. [DOI:10.1016/j.envint.2013.04.005] [PMID]
- Prata JC, da Costa JP, Lopes I, Duarte AC, Rocha-Santos T. Environmental exposure to microplastics: An overview on possible human health effects. Sci Total Environ. 2020; 702:134455. [DOI:10.1016/j.scitotenv.2019.134455] [PMID]
- Rist S, Carney Almroth B, Hartmann NB, Karlsson TM. A critical perspective on early communications concerning human health aspects of microplastics. Sci Total Environ. 2018; 626:720-6. [DOI:10.1016/j.scitotenv.2018.01.092] [PMID]
- Chain EPoCitF. Presence of microplastics and nanoplastics in food, with particular focus on seafood. Efsa J. 2016; 14(6):e04501. [DOI:10.2903/j.efsa.2016.4501]
Article Type: Narrative Review |
Subject:
Environmental Health
Received: 2024/09/12 | Accepted: 2024/11/10 | Published: 2025/01/1
Received: 2024/09/12 | Accepted: 2024/11/10 | Published: 2025/01/1
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |