Volume 10, Issue 3 (7-2025)
CJHR 2025, 10(3): 193-202 |
Back to browse issues page
Ethics code: IR. GUMS.1398.190
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Joukar F, Saghafi M, Yeganeh S, Hassanipour S, Mansour-Ghanaei F. Diagnostic Value of Faecal M2PK and FIT in Colorectal Cancer Screening. CJHR 2025; 10 (3) :193-202
URL: http://cjhr.gums.ac.ir/article-1-414-en.html
URL: http://cjhr.gums.ac.ir/article-1-414-en.html
Farahnaz Joukar1 
 , Mohammad Saghafi1
, Mohammad Saghafi1 
 , Sara Yeganeh1
, Sara Yeganeh1 
 , Soheil Hassanipour1
, Soheil Hassanipour1 
 , Fariborz Mansour-Ghanaei *2
, Fariborz Mansour-Ghanaei *2 


 , Mohammad Saghafi1
, Mohammad Saghafi1 
 , Sara Yeganeh1
, Sara Yeganeh1 
 , Soheil Hassanipour1
, Soheil Hassanipour1 
 , Fariborz Mansour-Ghanaei *2
, Fariborz Mansour-Ghanaei *2 

1- Gastrointestinal and Liver Diseases Research Center, Guilan University of Medical Sciences, Rasht, Iran
2- Gastrointestinal and Liver Diseases Research Center, Guilan University of Medical Sciences, Rasht, Iran ,fmansourghanaei@gmail.com
2- Gastrointestinal and Liver Diseases Research Center, Guilan University of Medical Sciences, Rasht, Iran ,
Full-Text [PDF 518 kb]
(340 Downloads)
| Abstract (HTML) (621 Views)
Full-Text: (349 Views)
Introduction
Colorectal cancer (CRC) is the third most common malignancy worldwide in both genders [1, 2]. According to GLOBOCAN 2020, CRC is the third most common cancer in Iran that is the fourth cancer in men and the second cancer in women [3]. Considering that CRC progresses from the adenoma stage to carcinoma in 10 to 15 years, early and regular screening is necessary to detect it in primary stage [4].
Generally, there are several methods for CRC screening, one of which is colonoscopy. Colonoscopy is a screening method with high sensitivity and specificity but most patients refuse to do it because of its invasive and painful nature [5, 6]. The non-invasive method includes the guaiac fecal occult blood test (gFOBT) and fecal immunochemical tests (FIT).
Today FIT is preferable to gFOBT because of having automatic analysis, higher sensitivity, and specificity for the early stages of cancer. FIT is a specific and direct measure of human hemoglobin in the stool. The basis of the test is based on monoclonal or polyclonal antibodies against human globin. FIT can be interpreted in two ways, a qualitative test based on visual indication by the immunochromatography method, and a quantitative test which measure hemoglobin numerically by the immunoturbidimetric methods and the values greater than the pre-defined threshold reported as positive. Therefore, diet does not affect it and it is more specific for lower gastrointestinal bleeding. Also, due to the possibility of automatic analysis and quantitative reporting in the form of micrograms of feces per gram, it is a non-invasive, cheap, and easy-to-perform method, which is accepted by most people as a suitable method [5-7]. The test sensitivity in CRC screening is relatively close to 70 to 90% [8, 9]. M2 pyruvate kinase (M2PK) is a biochemical test, which is much more convenient and more patient-friendly method with different performance when compared to FOBT [10]. The concentration of dimeric form of M2PK mainly increased in tumor cells. Measuring the level of this test in feces and determining its relationship with CRC was confirmed in many studies [6, 9, 11, 12]. M2PK is a pyruvate kinase enzyme that plays a key role in the conversion of phosphoenol pyruvate to pyruvate. This enzyme is very reactive, powerful, and forms of a tetramer. In the tumoral tissue that is exposed to oncoprotein, M2PK has become dimerized and weak, and this change is compulsory for tumor metabolism. In gastrointestinal tumors, M2PK is released into the gastrointestinal lumen, which is quickly detectable in feces. Therefore, due to the low sensitivity of FOBT, invasiveness of colonoscopy, high cost, and the unwillingness of people, we investigated the diagnostic efficacy of M2PK and FIT findings in clinical practice to have a suitable and correct method for modifying the prognosis and survival improvement of CRC. The objective of our study was to assess the M2PK and FIT tests in our clinical practice for primary screening of CRC due to their easy availability, ease of use, relatively low cost, and non-invasive nature as biomarkers.
Materials and Methods
Participants
This study was a cross-sectional analysis that was conducted on 768 people (384 people tested for M2PK, and 384 for FIT) referred to Guilan Gastroenterology and Liver Clinic in 2018, Rasht, Iran. A colonoscopy was performed on all new cases due to signs and symptoms in the colon without previous colonoscopies.
Demographic data
Demographic information filled out included age, sex, BMI, smoking and alcohol consumption by questionnaire. All data were collected by the physician through an interview. Inclusion criteria: All people with lower gastrointestinal symptoms over 18 years old. For instance, occult blood in the stool, hematochezia in the absence of a convincing anorectal source, melena (if the origin of the upper gastrointestinal system is not considered), people with iron deficiency. Exclusion criteria: History of polyps or colon cancer, people with known inflammatory bowel disease, having a positive family history of cancer and polyps, and pregnant women.
Clinical characteristics
Clinical characteristics including underlying diseases and gastrointestinal symptoms were obtained through interview with the patients. Stool samples were also taken from all people, and randomly allocated (1:1) using block randomization into two groups, 384 people were tested for M2PK and 384 for FIT. Finally, a colonoscopy was performed for both groups. M2PK: The sample volume required to perform this test was 100 mg. The stool samples were immediately sent to the laboratory center after collection. The sample should not be kept at room temperature for more than 48 hours and should be stored at -20 °C until testing. The M2PK was checked by the ELISA method (ScheBo kit, Germany) according to the manufacturer’s instruction. M2PK values greater than 4 U/ML were considered positive. FIT: Stool samples were collected. The FIT was performed immunologically (Vitrotec, Iran). The positive result (qualitative) of the test indicated abnormal bleeding in the lower digestive tract.
Colonoscopy
Colonoscopy was performed for both groups. The routine protocol of the colonoscopy section for bowel preparation was explained to the subjects by a trained interviewer. To monitor the implementation of the intervention and compliance with walking at the considered times according to the protocol, two telephone follow-ups were done the day before the colonoscopy. Biopsy specimens were evaluated and reviewed by two blinded expert pathologists.
Samples were analyzed and compared Kappa value (overall agreement) between the two pathologists was 0.96 (95% CI; 0.98%, 0.94%). The discrepancy between the pathologists was resolved by consensus or a third pathologist. Participants were divided into two groups according to colonoscopy and pathology findings group: 1. People with lesions group, 2. people without lesions. The result of the M2PK test and stool FIT were compared with colonoscopy and pathology findings in both groups.
Statistical analysis
Sensitivity, specificity, positive likelihood ratio, negative likelihood ratio, disease prevalence, positive predictive value, and negative predictive value were calculated using the statistical program MedCalc. Graphs were drawn using Prism software, version 7. We used a t-test for quantitative variables and a chi-square test for qualitative variables. A P<0.05 was considered statistically significant. Data analysis was performed using SPSSsoftware, version 23.
Results
Of the 768 participants, 54.7% were women. The mean age of participants was 51.5±14. 6 years. Demographic differences among the M2PK and FIT groups showed that in the FIT group, women had the largest population (57.3 %), and in the M2PK group, men had the largest population (52.1%). In both groups, most participants were in the age group of less than 50 years. Also, most participants had a BMI<25. Comparison of patients with and without colonoscopy lesions by demographic and clinical characteristics are described in Table 1.

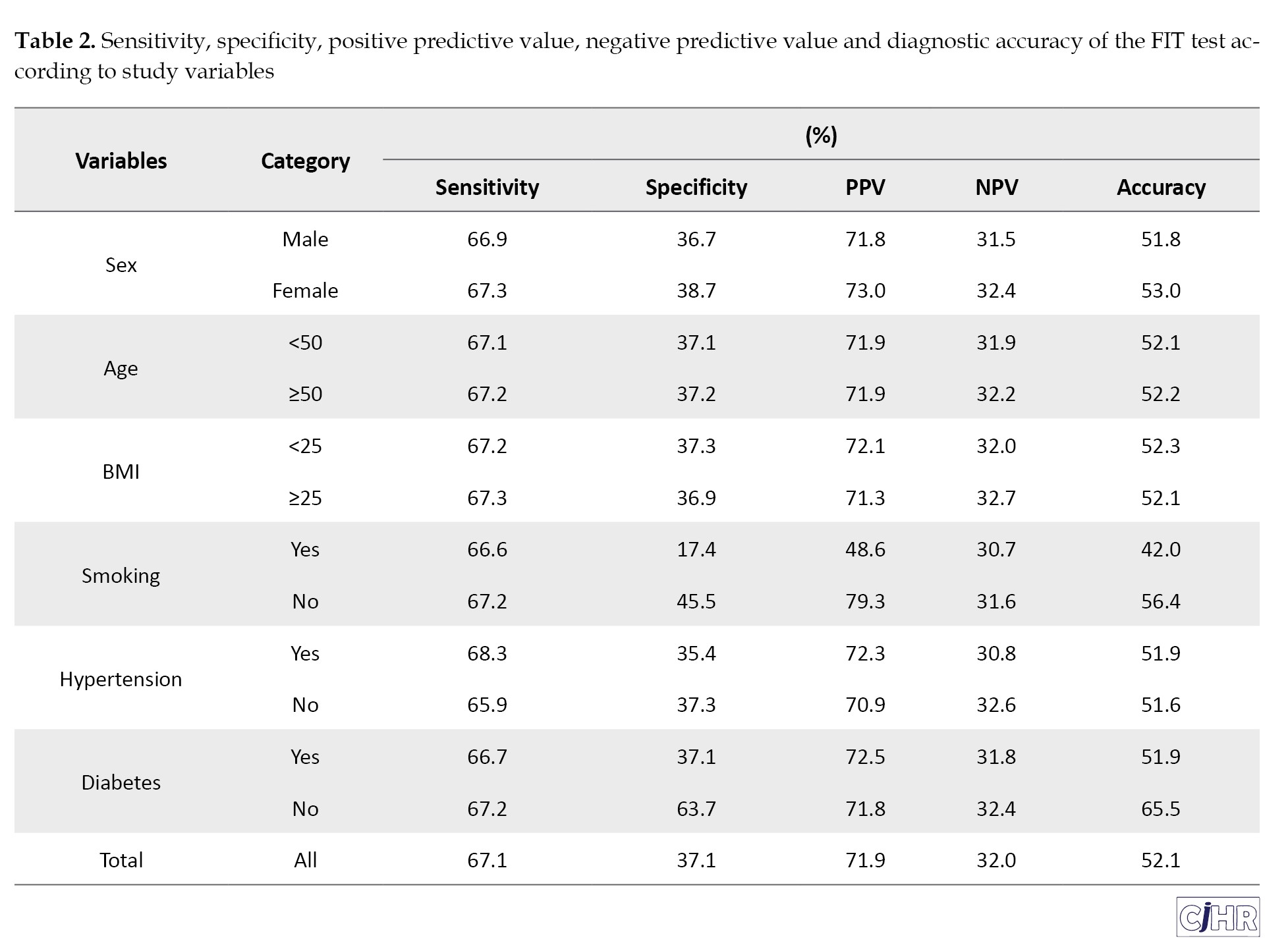
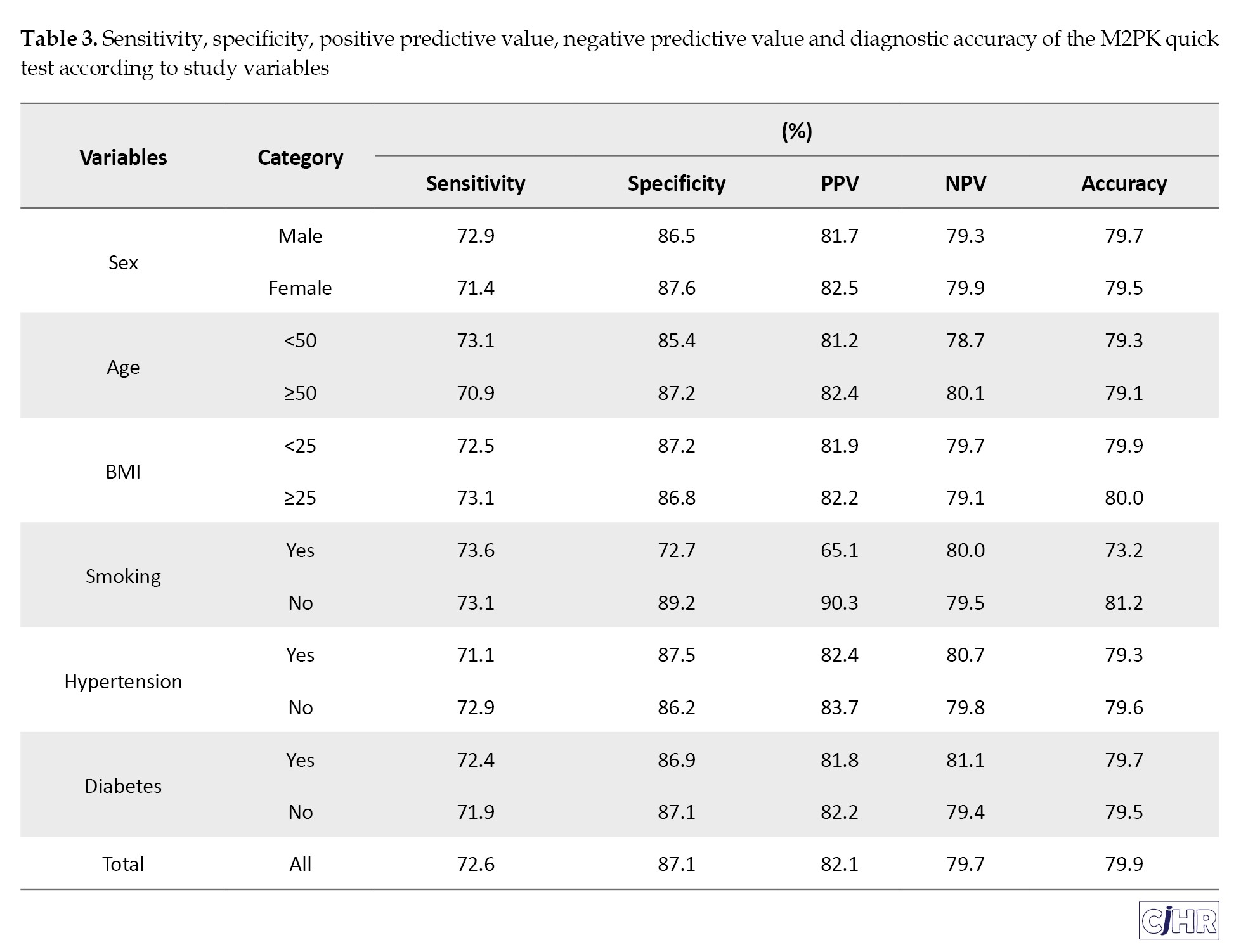
The sensitivity and specificity of the FIT were 67.1% and 37.1%, respectively, which were lower than those of M2PK test, with sensitivity and specificity of 72.6% and 87.1%, respectively. The of diagnostic values of the FIT and M2PK tests according to the characteristics of the participants are presented in Tables 2 and 3, respectively.
Discussion
CRC is one of the most common cancers of the gastrointestinal system [13]. Most people will not have any symptoms until the tumor is advanced. The survival rate depends on the stage of the tumor at the time of diagnosis. The 5-year survival rate in the early stages is 93% [10].
Non-invasive tests including FOBT (chemical assay), FIT (immunochemical assay), M2PK (immunochemical assay), and mt-s DNA (molecular assay- multitarget stool DNA test) are effective ways to identify early CRC and precancerous lesions for reducing mortality. Hence, screening programs reduce the disease burden. Colonoscopy is a sensitive and specific screening method but most patients refuse to undergo it due to its invasive and painful nature [14]. Parente et al. introduced the use of fecal markers FIT, M2PK, and calprotectin with high sensitivity and specificity in identifying colorectal neoplasia [15]. In Song et al. study, FIT was declared as an alternative test to FOBT with lower sensitivity, specificity, and cost [16]. In the study of Gutierrez-Stampa et al. and meta-analyses, it was stated that FIT should be used as a supportive diagnostic tool along with the clinical evaluation of patients [17-19]. In the study of Cruz et al. FIT in combination with M2PK in stool samples can be used in diagnosing malignancy and assisting the doctor in performing invasive procedures such as colonoscopy [20].
In this study, the sensitivity and specificity of FIT were 67.1% and 37.1%, respectively. In some studies, the specificity of FIT was reported to be 94%, which is not consistent with the present study [21, 22]. In the study of Song et al, the sensitivity of FIT was reported as 73.8% [16]. In this study, the negative predictive value of the FIT test was only 32.0%. The negative predictive value indicate percent of the cases that the test reported as negative and they are healthy. This index is the proportion of really healthy people. Since both healthy and sick cases are included in the calculation of these ratios the predictive value of a test in different societies with different prevalence values it will be different. In this way, whatever the prevalence of a disease if it is less, the negative predictive value of that diagnostic test will be higher. On the other hand, the higher the prevalence of a disease, the more positive the predictive value that test will be.
Various factors can affect the sensitivity and specificity of FIT. Age and gender can have significant effects on the interpretation of the FIT (fecal immunoassay) test for colon cancer. Studies have demonstrated that the sensitivity and specificity of this test vary across age and gender groups [23, 24]. The sensitivity of the FIT test is higher in older people. This increase in sensitivity may be due to the increase in hemoglobin concentration in the stool with age. At the same time, the specificity of the test may be slightly reduced in older people. In men, the sensitivity of the FIT test for detecting colon cancer is higher than in women. This difference may be due to the higher concentration of hemoglobin in men’s stool. Moreover, advanced-stage cancers are more commonly observed in men, which may influence the diagnostic sensitivity of FIT. To increase the accuracy of the FIT test in detecting colon cancer, it is suggested that different positive thresholds be used for different age and gender groups. This strategy may enhance the test’s accuracy by increasing sensitivity and specificity, thereby decreasing the frequency of unnecessary colonoscopy procedures [25, 26].
Studies have shown that the sensitivity of the FIT test varies across different areas of the colon and depends on the type of lesion [27, 28]. The impact of geographic region on the results of colon cancer screening using FIT varies significantly across countries. These differences are due to factors such as access to health services, regional health policies, demographic characteristics, and socioeconomic conditions. Challenges include recruiting participants, determining eligibility, and accessing colonoscopy at the appropriate time.
People who have previously participated in screening programs are less likely to have a positive result on the FIT test, as previous changes in intestinal tissue may have been detected [29]. Factors such as diet, physical activity, smoking, and alcohol consumption can affect FIT test results. For example, high consumption of fast food has been linked to an increased likelihood of a positive FIT test result [29].
Determination of M2PK sensitivity in this study was reported as 72.6%, which had a higher sensitivity than the FIT test. study was 64% and 84% respectively [10]. Zaccaro et al. illustrated that the combination of M2PK and FOBT will be effective in predicting CRC [30]. The specificity of M2PK was reported to be 87.1%, which had a higher specificity than the FIT test. The sensitivity and specificity of M2PK are consistent with the study of Uppara et al in England with a sensitivity and specificity of 79 % and 80% respectively [31]. Also, in the study of Hamzehzadeh et al. the sensitivity and specificity of M2PK were reported as 79% and 81%, which is consistent with our investigation [32]. In Aboelsoud et al study, the sensitivity and specificity of M2PK were 93% and 81%, respectively [33]. In Tonus et al. studies, the sensitivity and specificity of M2PK were reported as 81% and 95%, respectively [34]. Several other studies have pointed out the role of M2PK during cancer development, and its important role in neoplastic growth and glycolysis. Therefore, it is used as a marker to identify CRC [33, 35]. The sensitivity rate in Hardet et al, the study was 73% [36]. The sensitivity of the occult blood test in a study by 17. Gutierrez-Stampa et al. in people with CRC was reported as 24% [17].
In a meta-analysis, M2PK was suggested as a routine test for CRC screening [34]. In Dabbous et al. report, M2PK was superior test for differentiating CRC [37]. In the study of Shastri and his colleagues, FOBT test (sensitivity and specificity 64.5 and 96.3, respectively) compared to M2PK (sensitivity and specificity 72.4, 73.8 respectively) as a more common test, cheaper and faster was recommended for colorectal neoplasia [38]. In the study of Fung et al, the sensitivity and specificity of the FOBT test were higher than M2PK [39].
In the study of Vatandoost et al. DNA methylation evaluation tests and microRNA examination, along with stool tests such as FOBT, sigmoidoscopy, and colonoscopy, were the most accurate and sensitive, and the combination of screening tests was introduced as the best method [40].
The comparison of the FIT test with colonoscopy findings in the diagnosis of colon lesions showed that the demographic characteristics of people did not affect the diagnosis of the findings of these two tests.
Comparing the M2PK test with colonoscopy findings in terms of the presence of a lesion in demographic characteristics and co-morbidities, the results showed that there was a difference between the two tests in lesion detection only in terms of smoking. In the study by Ibrahim et al. no significant correlation was observed between tumor marker M2PK and age and gender [41]. In the Hamilton study, there was no correlation between M2PK and the age or gender of the patients [42]. Ibrahim et al. study and other studies agreed that higher M2PK was observed in patients with high BMI [41, 43, 44]. However, in the study of Haug et al, no correlation was observed between M2PK and BMI, and they acknowledged that no correlation was observed between BMI and colon cancer [14]. Also, the M2PK test was weaker in lesion location and 10 mm lesion size better than colonoscopy. There was no significant difference between the two tests in histological findings.
Comparison of demographic characteristics including sex, age, BMI, hypertension, diabetes in M2PK, FIT and colonoscopy was not significant (P>0.05).
Our study revealed that non-invasive CRC screening, like M2PK, is an alternative screening test for patients who do not want to undergo colonoscopy because of its reduced costs and high sensitivity and specificity. Although colonoscopy is the clear choice for the detection of CRC, M2PK is effective and cost-effective for primary screening of CRC.
Conclusion
The results of this study showed that the M2PK test had higher sensitivity and specificity compared to FIT. Therefore, this test can easily help in the recognition of colon cancer and intestinal polyps due to its higher sensitivity and specificity compared to FIT. As a result, M2PK is highly recommended for primary screening of CRC screening on a large scale due to its easy availability, easy-to-perform, relatively low cost, and non-invasive biomarker.
Strengthens and limitations
The strength of this research is that patients with colon cancer and those with a family history of colon cancer were excluded from the study, thereby minimizing confounding factors and reducing bias. Nonetheless, one of the limitations our study is that the time interval between symptom onset, testing, and diagnosis is unknown. On the other hand, FIT may have low sensitivity in detecting early-stage cancers and small tumors and only detect a high percentage of advanced cancers. FIT may also give false positive results in diseases such as IBD that cause bleeding in the stool which can affect the results. FIT can be used in large-scale CRC screening due to non-invasiveness, high patient comfort and acceptance, greater specificity than gFOBT, the possibility of performing it annually or at regular intervals, cost-effectiveness, and easy use. M2PK only indicates the potential presence of a lesion and is not capable of identifying its location, type, or informing treatment decisions.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Ethics Committee of Guilan University of Medical Sciences, Rasht, Iran (Code: IR. GUMS.1398.190). This study was conducted based on the Helsinki declaration and written consent form was obtained from the participants before conducting the study.
Funding
The paper was extracted from the fellow-ship thesis of the Mohammad Saghafi, approved by Guilan University of Medical Sciences, Rasht, Iran.
Authors' contributions
All authors contributed equally to the conception and design of the study, data collection and analysis, interpretation of the results, and drafting of the manuscript. Each author approved the final version of the manuscript for submission.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgements
The authors would like to express their sincere gratitude to all participants in this study, as well as to the dedicated staff of the Guilan Gastrointestinal and Liver Diseases Research Center, for their invaluable support and cooperation.
Colorectal cancer (CRC) is the third most common malignancy worldwide in both genders [1, 2]. According to GLOBOCAN 2020, CRC is the third most common cancer in Iran that is the fourth cancer in men and the second cancer in women [3]. Considering that CRC progresses from the adenoma stage to carcinoma in 10 to 15 years, early and regular screening is necessary to detect it in primary stage [4].
Generally, there are several methods for CRC screening, one of which is colonoscopy. Colonoscopy is a screening method with high sensitivity and specificity but most patients refuse to do it because of its invasive and painful nature [5, 6]. The non-invasive method includes the guaiac fecal occult blood test (gFOBT) and fecal immunochemical tests (FIT).
Today FIT is preferable to gFOBT because of having automatic analysis, higher sensitivity, and specificity for the early stages of cancer. FIT is a specific and direct measure of human hemoglobin in the stool. The basis of the test is based on monoclonal or polyclonal antibodies against human globin. FIT can be interpreted in two ways, a qualitative test based on visual indication by the immunochromatography method, and a quantitative test which measure hemoglobin numerically by the immunoturbidimetric methods and the values greater than the pre-defined threshold reported as positive. Therefore, diet does not affect it and it is more specific for lower gastrointestinal bleeding. Also, due to the possibility of automatic analysis and quantitative reporting in the form of micrograms of feces per gram, it is a non-invasive, cheap, and easy-to-perform method, which is accepted by most people as a suitable method [5-7]. The test sensitivity in CRC screening is relatively close to 70 to 90% [8, 9]. M2 pyruvate kinase (M2PK) is a biochemical test, which is much more convenient and more patient-friendly method with different performance when compared to FOBT [10]. The concentration of dimeric form of M2PK mainly increased in tumor cells. Measuring the level of this test in feces and determining its relationship with CRC was confirmed in many studies [6, 9, 11, 12]. M2PK is a pyruvate kinase enzyme that plays a key role in the conversion of phosphoenol pyruvate to pyruvate. This enzyme is very reactive, powerful, and forms of a tetramer. In the tumoral tissue that is exposed to oncoprotein, M2PK has become dimerized and weak, and this change is compulsory for tumor metabolism. In gastrointestinal tumors, M2PK is released into the gastrointestinal lumen, which is quickly detectable in feces. Therefore, due to the low sensitivity of FOBT, invasiveness of colonoscopy, high cost, and the unwillingness of people, we investigated the diagnostic efficacy of M2PK and FIT findings in clinical practice to have a suitable and correct method for modifying the prognosis and survival improvement of CRC. The objective of our study was to assess the M2PK and FIT tests in our clinical practice for primary screening of CRC due to their easy availability, ease of use, relatively low cost, and non-invasive nature as biomarkers.
Materials and Methods
Participants
This study was a cross-sectional analysis that was conducted on 768 people (384 people tested for M2PK, and 384 for FIT) referred to Guilan Gastroenterology and Liver Clinic in 2018, Rasht, Iran. A colonoscopy was performed on all new cases due to signs and symptoms in the colon without previous colonoscopies.
Demographic data
Demographic information filled out included age, sex, BMI, smoking and alcohol consumption by questionnaire. All data were collected by the physician through an interview. Inclusion criteria: All people with lower gastrointestinal symptoms over 18 years old. For instance, occult blood in the stool, hematochezia in the absence of a convincing anorectal source, melena (if the origin of the upper gastrointestinal system is not considered), people with iron deficiency. Exclusion criteria: History of polyps or colon cancer, people with known inflammatory bowel disease, having a positive family history of cancer and polyps, and pregnant women.
Clinical characteristics
Clinical characteristics including underlying diseases and gastrointestinal symptoms were obtained through interview with the patients. Stool samples were also taken from all people, and randomly allocated (1:1) using block randomization into two groups, 384 people were tested for M2PK and 384 for FIT. Finally, a colonoscopy was performed for both groups. M2PK: The sample volume required to perform this test was 100 mg. The stool samples were immediately sent to the laboratory center after collection. The sample should not be kept at room temperature for more than 48 hours and should be stored at -20 °C until testing. The M2PK was checked by the ELISA method (ScheBo kit, Germany) according to the manufacturer’s instruction. M2PK values greater than 4 U/ML were considered positive. FIT: Stool samples were collected. The FIT was performed immunologically (Vitrotec, Iran). The positive result (qualitative) of the test indicated abnormal bleeding in the lower digestive tract.
Colonoscopy
Colonoscopy was performed for both groups. The routine protocol of the colonoscopy section for bowel preparation was explained to the subjects by a trained interviewer. To monitor the implementation of the intervention and compliance with walking at the considered times according to the protocol, two telephone follow-ups were done the day before the colonoscopy. Biopsy specimens were evaluated and reviewed by two blinded expert pathologists.
Samples were analyzed and compared Kappa value (overall agreement) between the two pathologists was 0.96 (95% CI; 0.98%, 0.94%). The discrepancy between the pathologists was resolved by consensus or a third pathologist. Participants were divided into two groups according to colonoscopy and pathology findings group: 1. People with lesions group, 2. people without lesions. The result of the M2PK test and stool FIT were compared with colonoscopy and pathology findings in both groups.
Statistical analysis
Sensitivity, specificity, positive likelihood ratio, negative likelihood ratio, disease prevalence, positive predictive value, and negative predictive value were calculated using the statistical program MedCalc. Graphs were drawn using Prism software, version 7. We used a t-test for quantitative variables and a chi-square test for qualitative variables. A P<0.05 was considered statistically significant. Data analysis was performed using SPSSsoftware, version 23.
Results
Of the 768 participants, 54.7% were women. The mean age of participants was 51.5±14. 6 years. Demographic differences among the M2PK and FIT groups showed that in the FIT group, women had the largest population (57.3 %), and in the M2PK group, men had the largest population (52.1%). In both groups, most participants were in the age group of less than 50 years. Also, most participants had a BMI<25. Comparison of patients with and without colonoscopy lesions by demographic and clinical characteristics are described in Table 1.

The sensitivity and specificity of the FIT were 67.1% and 37.1%, respectively, which were lower than those of M2PK test, with sensitivity and specificity of 72.6% and 87.1%, respectively. The of diagnostic values of the FIT and M2PK tests according to the characteristics of the participants are presented in Tables 2 and 3, respectively.


Discussion
CRC is one of the most common cancers of the gastrointestinal system [13]. Most people will not have any symptoms until the tumor is advanced. The survival rate depends on the stage of the tumor at the time of diagnosis. The 5-year survival rate in the early stages is 93% [10].
Non-invasive tests including FOBT (chemical assay), FIT (immunochemical assay), M2PK (immunochemical assay), and mt-s DNA (molecular assay- multitarget stool DNA test) are effective ways to identify early CRC and precancerous lesions for reducing mortality. Hence, screening programs reduce the disease burden. Colonoscopy is a sensitive and specific screening method but most patients refuse to undergo it due to its invasive and painful nature [14]. Parente et al. introduced the use of fecal markers FIT, M2PK, and calprotectin with high sensitivity and specificity in identifying colorectal neoplasia [15]. In Song et al. study, FIT was declared as an alternative test to FOBT with lower sensitivity, specificity, and cost [16]. In the study of Gutierrez-Stampa et al. and meta-analyses, it was stated that FIT should be used as a supportive diagnostic tool along with the clinical evaluation of patients [17-19]. In the study of Cruz et al. FIT in combination with M2PK in stool samples can be used in diagnosing malignancy and assisting the doctor in performing invasive procedures such as colonoscopy [20].
In this study, the sensitivity and specificity of FIT were 67.1% and 37.1%, respectively. In some studies, the specificity of FIT was reported to be 94%, which is not consistent with the present study [21, 22]. In the study of Song et al, the sensitivity of FIT was reported as 73.8% [16]. In this study, the negative predictive value of the FIT test was only 32.0%. The negative predictive value indicate percent of the cases that the test reported as negative and they are healthy. This index is the proportion of really healthy people. Since both healthy and sick cases are included in the calculation of these ratios the predictive value of a test in different societies with different prevalence values it will be different. In this way, whatever the prevalence of a disease if it is less, the negative predictive value of that diagnostic test will be higher. On the other hand, the higher the prevalence of a disease, the more positive the predictive value that test will be.
Various factors can affect the sensitivity and specificity of FIT. Age and gender can have significant effects on the interpretation of the FIT (fecal immunoassay) test for colon cancer. Studies have demonstrated that the sensitivity and specificity of this test vary across age and gender groups [23, 24]. The sensitivity of the FIT test is higher in older people. This increase in sensitivity may be due to the increase in hemoglobin concentration in the stool with age. At the same time, the specificity of the test may be slightly reduced in older people. In men, the sensitivity of the FIT test for detecting colon cancer is higher than in women. This difference may be due to the higher concentration of hemoglobin in men’s stool. Moreover, advanced-stage cancers are more commonly observed in men, which may influence the diagnostic sensitivity of FIT. To increase the accuracy of the FIT test in detecting colon cancer, it is suggested that different positive thresholds be used for different age and gender groups. This strategy may enhance the test’s accuracy by increasing sensitivity and specificity, thereby decreasing the frequency of unnecessary colonoscopy procedures [25, 26].
Studies have shown that the sensitivity of the FIT test varies across different areas of the colon and depends on the type of lesion [27, 28]. The impact of geographic region on the results of colon cancer screening using FIT varies significantly across countries. These differences are due to factors such as access to health services, regional health policies, demographic characteristics, and socioeconomic conditions. Challenges include recruiting participants, determining eligibility, and accessing colonoscopy at the appropriate time.
People who have previously participated in screening programs are less likely to have a positive result on the FIT test, as previous changes in intestinal tissue may have been detected [29]. Factors such as diet, physical activity, smoking, and alcohol consumption can affect FIT test results. For example, high consumption of fast food has been linked to an increased likelihood of a positive FIT test result [29].
Determination of M2PK sensitivity in this study was reported as 72.6%, which had a higher sensitivity than the FIT test. study was 64% and 84% respectively [10]. Zaccaro et al. illustrated that the combination of M2PK and FOBT will be effective in predicting CRC [30]. The specificity of M2PK was reported to be 87.1%, which had a higher specificity than the FIT test. The sensitivity and specificity of M2PK are consistent with the study of Uppara et al in England with a sensitivity and specificity of 79 % and 80% respectively [31]. Also, in the study of Hamzehzadeh et al. the sensitivity and specificity of M2PK were reported as 79% and 81%, which is consistent with our investigation [32]. In Aboelsoud et al study, the sensitivity and specificity of M2PK were 93% and 81%, respectively [33]. In Tonus et al. studies, the sensitivity and specificity of M2PK were reported as 81% and 95%, respectively [34]. Several other studies have pointed out the role of M2PK during cancer development, and its important role in neoplastic growth and glycolysis. Therefore, it is used as a marker to identify CRC [33, 35]. The sensitivity rate in Hardet et al, the study was 73% [36]. The sensitivity of the occult blood test in a study by 17. Gutierrez-Stampa et al. in people with CRC was reported as 24% [17].
In a meta-analysis, M2PK was suggested as a routine test for CRC screening [34]. In Dabbous et al. report, M2PK was superior test for differentiating CRC [37]. In the study of Shastri and his colleagues, FOBT test (sensitivity and specificity 64.5 and 96.3, respectively) compared to M2PK (sensitivity and specificity 72.4, 73.8 respectively) as a more common test, cheaper and faster was recommended for colorectal neoplasia [38]. In the study of Fung et al, the sensitivity and specificity of the FOBT test were higher than M2PK [39].
In the study of Vatandoost et al. DNA methylation evaluation tests and microRNA examination, along with stool tests such as FOBT, sigmoidoscopy, and colonoscopy, were the most accurate and sensitive, and the combination of screening tests was introduced as the best method [40].
The comparison of the FIT test with colonoscopy findings in the diagnosis of colon lesions showed that the demographic characteristics of people did not affect the diagnosis of the findings of these two tests.
Comparing the M2PK test with colonoscopy findings in terms of the presence of a lesion in demographic characteristics and co-morbidities, the results showed that there was a difference between the two tests in lesion detection only in terms of smoking. In the study by Ibrahim et al. no significant correlation was observed between tumor marker M2PK and age and gender [41]. In the Hamilton study, there was no correlation between M2PK and the age or gender of the patients [42]. Ibrahim et al. study and other studies agreed that higher M2PK was observed in patients with high BMI [41, 43, 44]. However, in the study of Haug et al, no correlation was observed between M2PK and BMI, and they acknowledged that no correlation was observed between BMI and colon cancer [14]. Also, the M2PK test was weaker in lesion location and 10 mm lesion size better than colonoscopy. There was no significant difference between the two tests in histological findings.
Comparison of demographic characteristics including sex, age, BMI, hypertension, diabetes in M2PK, FIT and colonoscopy was not significant (P>0.05).
Our study revealed that non-invasive CRC screening, like M2PK, is an alternative screening test for patients who do not want to undergo colonoscopy because of its reduced costs and high sensitivity and specificity. Although colonoscopy is the clear choice for the detection of CRC, M2PK is effective and cost-effective for primary screening of CRC.
Conclusion
The results of this study showed that the M2PK test had higher sensitivity and specificity compared to FIT. Therefore, this test can easily help in the recognition of colon cancer and intestinal polyps due to its higher sensitivity and specificity compared to FIT. As a result, M2PK is highly recommended for primary screening of CRC screening on a large scale due to its easy availability, easy-to-perform, relatively low cost, and non-invasive biomarker.
Strengthens and limitations
The strength of this research is that patients with colon cancer and those with a family history of colon cancer were excluded from the study, thereby minimizing confounding factors and reducing bias. Nonetheless, one of the limitations our study is that the time interval between symptom onset, testing, and diagnosis is unknown. On the other hand, FIT may have low sensitivity in detecting early-stage cancers and small tumors and only detect a high percentage of advanced cancers. FIT may also give false positive results in diseases such as IBD that cause bleeding in the stool which can affect the results. FIT can be used in large-scale CRC screening due to non-invasiveness, high patient comfort and acceptance, greater specificity than gFOBT, the possibility of performing it annually or at regular intervals, cost-effectiveness, and easy use. M2PK only indicates the potential presence of a lesion and is not capable of identifying its location, type, or informing treatment decisions.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Ethics Committee of Guilan University of Medical Sciences, Rasht, Iran (Code: IR. GUMS.1398.190). This study was conducted based on the Helsinki declaration and written consent form was obtained from the participants before conducting the study.
Funding
The paper was extracted from the fellow-ship thesis of the Mohammad Saghafi, approved by Guilan University of Medical Sciences, Rasht, Iran.
Authors' contributions
All authors contributed equally to the conception and design of the study, data collection and analysis, interpretation of the results, and drafting of the manuscript. Each author approved the final version of the manuscript for submission.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgements
The authors would like to express their sincere gratitude to all participants in this study, as well as to the dedicated staff of the Guilan Gastrointestinal and Liver Diseases Research Center, for their invaluable support and cooperation.
References
- Rawla P, Sunkara T, Barsouk A. Epidemiology of colorectal cancer: incidence, mortality, survival, and risk factors. Prz Gastroenterol. 2019; 14(2):89-103. [DOI:10.5114/pg.2018.81072] [PMID]
- Xi Y, Xu P. Global colorectal cancer burden in 2020 and projections to 2040. Transl Oncol. 2021; 14(10):101174. [DOI:10.1016/j.tranon.2021.101174] [PMID]
- International Agency for Research on Cancer. Data visualization tools for exploring the global cancer burden in 2022. 2022 [Updated 2025 July 29]. Available from: [Link]
- Molfino A, Formiconi A, Leone PM, Rossi Fanelli F, Muscaritoli M. Towards improved awareness and earlier diagnosis of early onset colorectal neoplasms. Intern Emerg Med. 2014; 9(6):615-6. [DOI:10.1007/s11739-014-1101-8] [PMID]
- Niederreiter M, Niederreiter L, Schmiderer A, Tilg H, Djanani A. Colorectal cancer screening and prevention-Pros and cons. Memo Mag Eur Med. Oncol. 2019; 12:239-43. [DOI:10.1007/s12254-019-00520-z]
- Anderson RD, Patel R, Hamilton JK, Boland CR. Cronkhite-Canada syndrome presenting as eosinophilic gastroenteritis. Proc (Bayl Univ Med Cent). 2006; 19(3):209-12. [DOI:10.1080/08998280.2006.11928163] [PMID]
- La Torre G, Miele L, Mannocci A, Chiaradia G, Berloco F, Gabrieli ML, et al. Correlates of HCV seropositivity among familial contacts of HCV positive patients. BMc Public Health. 2006; 6(1):237. [DOI:10.1186/1471-2458-6-237]
- Niedermaier T, Balavarca Y, Brenner H. Stage-specific sensitivity of fecal immunochemical tests for detecting colorectal cancer: Systematic review and meta-analysis. Am J Gastroenterol. 2020; 115(1):56-69. [DOI:10.14309/ajg.0000000000000465] [PMID]
- Sweetser S, Ahlquist DA, Osborn NK, Sanderson SO, Smyrk TC, Chari ST, et al. Clinicopathologic features and treatment outcomes in Cronkhite-Canada syndrome: Support for autoimmunity. Dig Dis Sci. 2012; 57:496-502. [DOI:10.1007/s10620-011-1874-9] [PMID]
- Ghaffari SYD, Azhogh R. Diagnostic accuracy of m2 pyruvate kinase quick stool test and fecal occult blood test for detection of colorectal cancer. Med J Tabriz Univ Med Sci. 2020; 42(3):287-94. [DOI:10.34172/mj.2020.047]
- Idigoras I, Arrospide A, Portillo I, Arana-Arri E, Martínez-Indart L, Mar J, et al. Evaluation of the colorectal cancer screening Programme in the Basque Country (Spain) and its effectiveness based on the Miscan-colon model. BMC Public Health. 2017; 18(1):78. [DOI:10.1186/s12889-017-4639-3] [PMID]
- Zbuk KM, Eng C. Hamartomatous polyposis syndromes. Nat Clin Pract Gastroenterol Hepatol. 2007; 4(9):492-502. [DOI:10.1038/ncpgasthep0902] [PMID]
- Brunicardi FC, Andersen D, Billiar T, Dunn DL, Hunter JG, Kao LS, et al. Schwartz’s principles of surgery. Columbus: McGrow Hill; 2005. [Link]
- Haug U, Rothenbacher D, Wente MN, Seiler CM, Stegmaier C, Brenner H. Tumour M2-PK as a stool marker for colorectal cancer: Comparative analysis in a large sample of unselected older adults vs colorectal cancer patients. Br J Cancer. 2007; 96(9):1329-34. [DOI:10.1038/sj.bjc.6603712] [PMID]
- Parente F, Marino B, Ilardo A, Fracasso P, Zullo A, Hassan C, et al. A combination of faecal tests for the detection of colon cancer: A new strategy for an appropriate selection of referrals to colonoscopy? A prospective multicentre Italian study. Eur J Gastroenterol Hepatol. 2012; 24(10):1145-52. [DOI:10.1097/MEG.0b013e328355cc79] [PMID]
- Song LL, Li YM. Current noninvasive tests for colorectal cancer screening: An overview of colorectal cancer screening tests. World J Gastrointest Oncol. 2016; 8(11):793-800. [DOI:10.4251/wjgo.v8.i11.793] [PMID]
- Gutierrez-Stampa MA, Aguilar V, Sarasqueta C, Cubiella J, Portillo I, Bujanda L. Impact of the faecal immunochemical test on colorectal cancer survival. BMC Cancer. 2020; 20(1):616. [DOI:10.1186/s12885-020-07074-y] [PMID]
- Pin Vieito N, Zarraquiños S, Cubiella J. High-risk symptoms and quantitative faecal immunochemical test accuracy: Systematic review and meta-analysis. World J Gastroenterol. 2019; 25(19):2383-401. [DOI:10.3748/wjg.v25.i19.2383] [PMID]
- Westwood M, Lang S, Armstrong N, van Turenhout S, Cubiella J, Stirk L, et al. Faecal immunochemical tests (FIT) can help to rule out colorectal cancer in patients presenting in primary care with lower abdominal symptoms: A systematic review conducted to inform new NICE DG30 diagnostic guidance. BMC Med. 2017; 15(1):189. [DOI:10.1186/s12916-017-0944-z] [PMID]
- Cruz A, Carvalho CM, Cunha A, Crespo A, Iglesias Á, García-Nimo L, et al. Faecal diagnostic biomarkers for colorectal cancer. Cancers. 2021; 13(21):5568. [DOI:10.3390/cancers13215568] [PMID]
- Imperiale TF, Ransohoff DF, Itzkowitz SH, Turnbull BA, Ross ME; Colorectal Cancer Study Group. Fecal DNA versus fecal occult blood for colorectal-cancer screening in an average-risk population. N Engl J Med. 2004; 351(26):2704-14. [DOI:10.1056/NEJMoa033403] [PMID]
- Lieberman DA, Weiss DG; Veterans Affairs Cooperative Study Group 380. One-time screening for colorectal cancer with combined fecal occult-blood testing and examination of the distal colon. N Engl J Med. 2001; 345(8):555-60. [DOI:10.1056/NEJMoa010328] [PMID]
- Mobarak S, Salasi M, Hormati A, Khodadadi J, Ziaee M, Abedi F, et al. Evaluation of the effect of sofosbuvir and daclatasvir in hospitalized COVID-19 patients: A randomized double-blind clinical trial (DISCOVER). J Antimicrob Chemother. 2022; 77(3):758-66. [DOI:10.1093/jac/dkab433] [PMID]
- Njor SH, Rasmussen M, Friis-Hansen L, Andersen B. Varying fecal immunochemical test screening cutoffs by age and gender: A way to increase detection rates and reduce the number of colonoscopies. Gastrointest Endosc. 2022; 95(3):540-9. [DOI:10.1016/j.gie.2021.09.038] [PMID]
- Brenner H, Qian J, Werner S. Variation of diagnostic performance of fecal immunochemical testing for hemoglobin by sex and age: Results from a large screening cohort. Clin Epidemiol. 2018; 10:381-9. [DOI:10.2147/CLEP.S155548] [PMID]
- Selby K, Levine EH, Doan C, Gies A, Brenner H, Quesenberry C, et al. Effect of sex, age, and positivity threshold on fecal immunochemical test accuracy: A systematic review and meta-analysis. Gastroenterology. 2019; 157(6):1494-505. [DOI:10.1053/j.gastro.2019.08.023] [PMID]
- Niedermaier T, Tikk K, Gies A, Bieck S, Brenner H. Sensitivity of fecal immunochemical test for colorectal cancer detection differs according to stage and location. Clin Gastroenterol Hepatol. 2020; 18(13):2920-8.e6. [DOI:10.1016/j.cgh.2020.01.025] [PMID]
- Brenner H, Niedermaier T, Chen H. Strong subsite-specific variation in detecting advanced adenomas by fecal immunochemical testing for hemoglobin. Int J Cancer. 2017; 140(9):2015-22. [DOI:10.1002/ijc.30629] [PMID]
- Symonds EL, Osborne JM, Cole SR, Bampton PA, Fraser RJ, Young GP. Factors affecting faecal immunochemical test positive rates: Demographic, pathological, behavioural and environmental variables. J Med Screen. 2015; 22(4):187-93. [DOI:10.1177/0969141315584783] [PMID]
- Zaccaro C, Saracino IM, Fiorini G, Figura N, Holton J, Castelli V, et al. Power of screening tests for colorectal cancer enhanced by high levels of M2-PK in addition to FOBT. Intern Emerg Med. 2017; 12(3):333-9. [DOI:10.1007/s11739-017-1610-3] [PMID]
- Uppara M, Adaba F, Askari A, Clark S, Hanna G, Athanasiou T, et al. A systematic review and meta-analysis of the diagnostic accuracy of pyruvate kinase M2 isoenzymatic assay in diagnosing colorectal cancer. World J Surg Oncol. 2015; 13:48. [DOI:10.1186/s12957-015-0446-4] [PMID]
- Hamzehzadeh L, Yousefi M, Ghaffari SH. Colorectal cancer screening: A comprehensive review to recent non-invasive methods. Int J Hematol Oncol Stem Cell Res. 2017; 11(3):250. [PMID]
- Talaat A, Aboelsoud A, Elehleh A, Montser B, El Khayat M. Study of the diagnostic role of fecal M2-pyruvate kinase level in patients with colorectal cancer. Menoufia Med J. 2021; 34(4):1268-74. [Link]
- Tonus C, Sellinger M, Koss K, Neupert G. Faecal pyruvate kinase isoenzyme type M2 for colorectal cancer screening: A meta-analysis. World J Gastroenterol. 2012; 18(30):4004-11. [DOI:10.3748/wjg.v18.i30.4004] [PMID]
- Hassan AA, Bastawy M, El-Hawary M, Soliman K. Evaluation of fecal pyrovatekinase isoenzyme (M2-PK) level in differetiating functional from organic colonic disorders. Al-Azhar Med J. 2017; 46(1):183-92. [DOI:10.12816/0035547]
- Hardt P, Ngoumou B, Rupp J, Schnell-Kretschmer H, Kloer H. Tumor M2-pyruvate kinase: A promising tumor marker in the diagnosis of gastro-intestinal cancer. Anticancer Res. 2000; 20(6D):4965-8. [DOI:10.1016/S0016-5085(00)83115-7]
- Dabbous HK, Mohamed YAE, El-Folly RF, El-Talkawy MD, Seddik HE, Johar D, et al. Evaluation of fecal M2PK as a Diagnostic Marker in Colorectal Cancer. J Gastrointest Cancer. 2019; 50(3):442-50. [DOI:10.1007/s12029-018-0088-1] [PMID]
- Shastri YM, Naumann M, Oremek GM, Hanisch E, Rösch W, Mössner J, et al. Prospective multicenter evaluation of fecal tumor pyruvate kinase type M2 (M2-PK) as a screening biomarker for colorectal neoplasia. Int J Cancer. 2006; 119(11):2651-6. [DOI:10.1002/ijc.22243] [PMID]
- Fung TT, Van Dam RM, Hankinson SE, Stampfer M, Willett WC, Hu FB. Low-carbohydrate diets and all-cause and cause-specific mortality: two cohort studies. Ann Intern Med. 2010; 153:289-98. [DOI:10.7326/0003-4819-153-5-201009070-00003] [PMID]
- Vatandoost N, Ghanbari J, Mojaver M, Avan A, Ghayour-Mobarhan M, et al. Early detection of colorectal cancer: From conventional methods to novel biomarkers. J Cancer Res Clin Oncol. 2016; 142(2):341-51. [DOI:10.1007/s00432-015-1928-z] [PMID]
- Ibrahim MET, Mohamed MA, Elawady MA, Abed HA. The fecal M2-PK as a novel biomarker for screening of cancer colon. Egypt J Commun Med. 2018; 36(3):93-102. [DOI:10.21608/ejcm.2018.16337]
- Bosman FT CF, Hruban RH, Theise ND. WHO classification of tumours of the digestive system. Geneva: World Health Organization. 2010. [Link]
- Kim MC, Kim CS, Chung TH, Park HO, Yoo CI. Metabolic syndrome, lifestyle risk factors, and distal colon adenoma: a retrospective cohort study. World J Gastroenterol. 2011; 17(35):4031-7. [DOI:10.3748/wjg.v17.i35.4031] [PMID]
- Levi Z, Kark JD, Barchana M, Liphshitz I, Zavdy O, Tzur D, et al. Measured body mass index in adolescence and the incidence of colorectal cancer in a cohort of 1.1 million males. Cancer Epidemiol Biomarkers Prev. 2011; 20(12):2524-31. [DOI:10.1158/1055-9965.EPI-11-0531] [PMID]
Article Type: Original Contributions |
Subject:
Public Health
Received: 2025/04/7 | Accepted: 2025/05/20 | Published: 2025/07/1
Received: 2025/04/7 | Accepted: 2025/05/20 | Published: 2025/07/1
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |






